Anat Cell Biol.
2010 Mar;43(1):86-95. 10.5115/acb.2010.43.1.86.
Early cerebellar granule cell migration in the mouse embryonic development
- Affiliations
-
- 1Department of Cell Biology and Human Anatomy, School of Medicine, University of California, Davis, Sacramento, California 95817, USA.
- 2Department of Anatomy, College of Medicine, Konyang University, Daejeon, Korea. ygjeong@konyang.ac.kr
- 3Department of Companion Animal and Animal Resources Science, Joongbu University, Geumsan-gun, Korea.
- KMID: 2168901
- DOI: http://doi.org/10.5115/acb.2010.43.1.86
Abstract
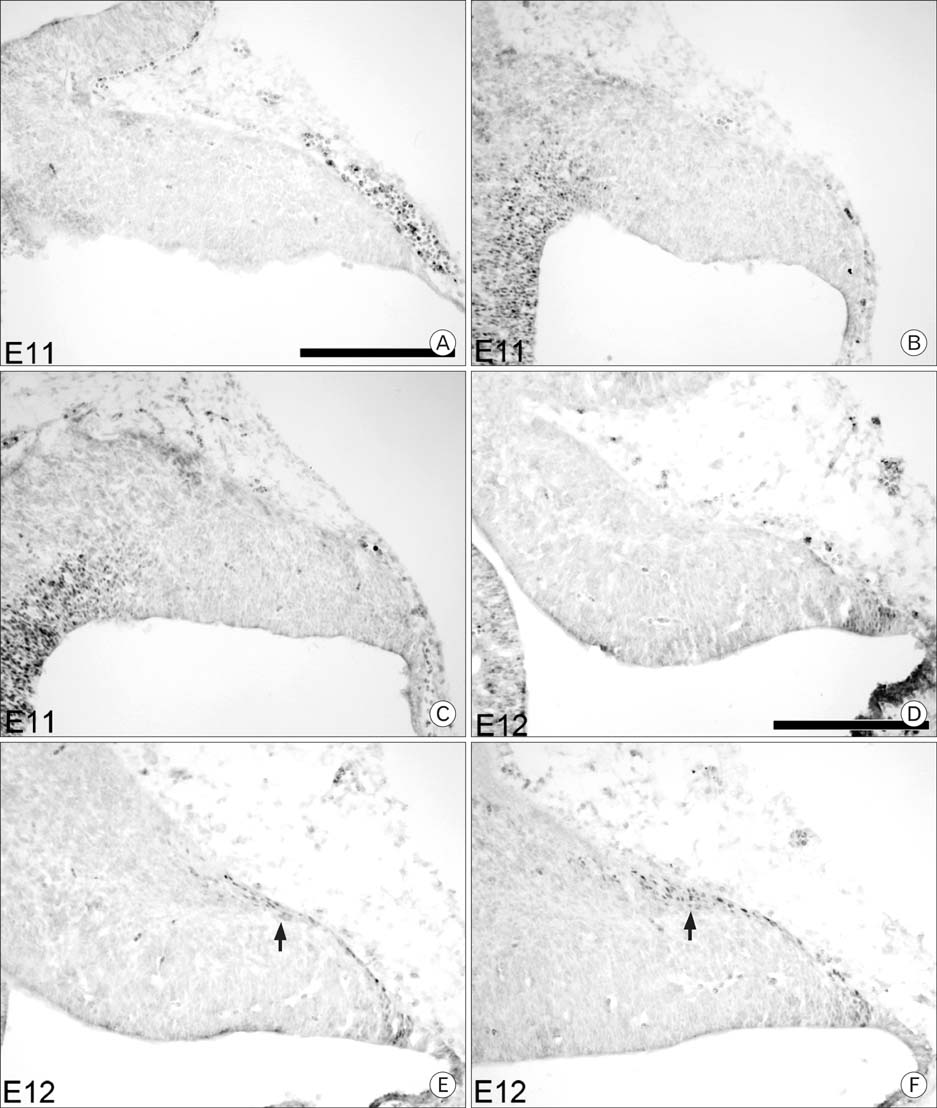
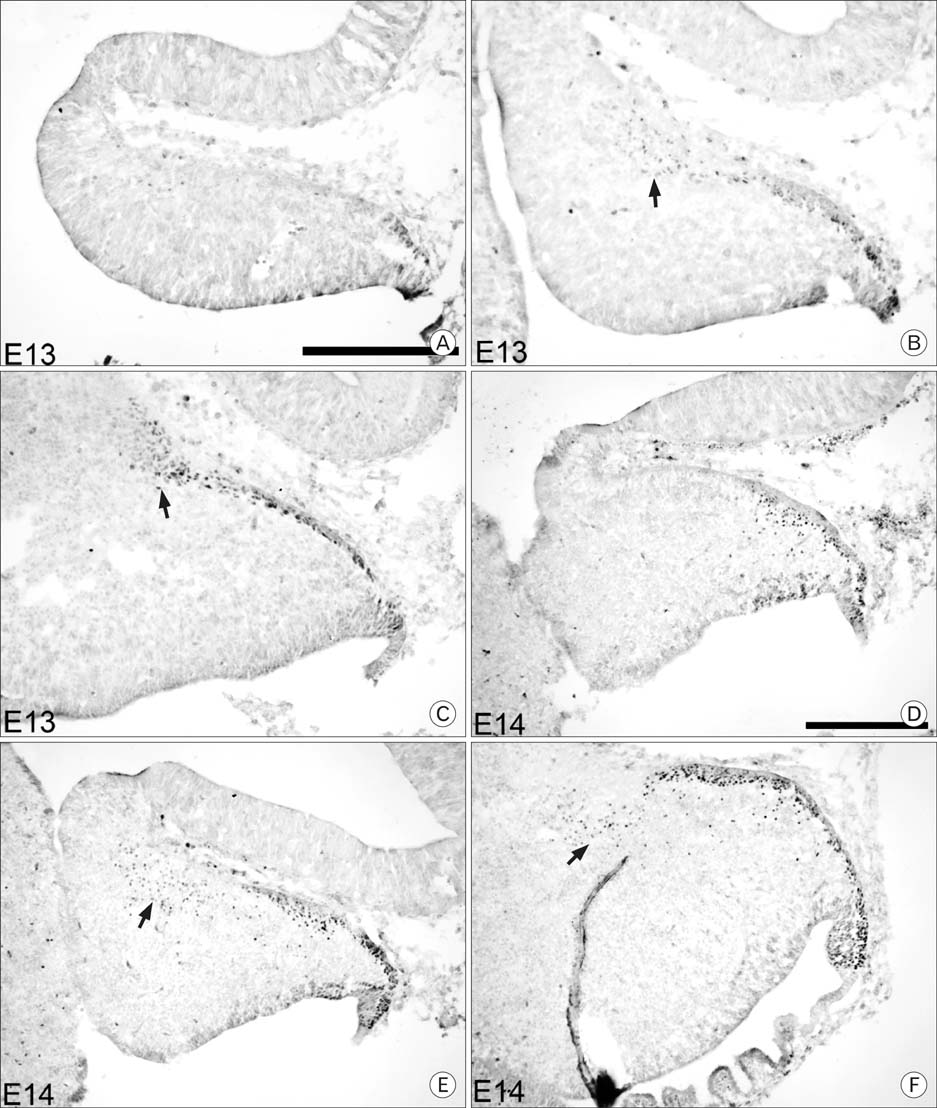
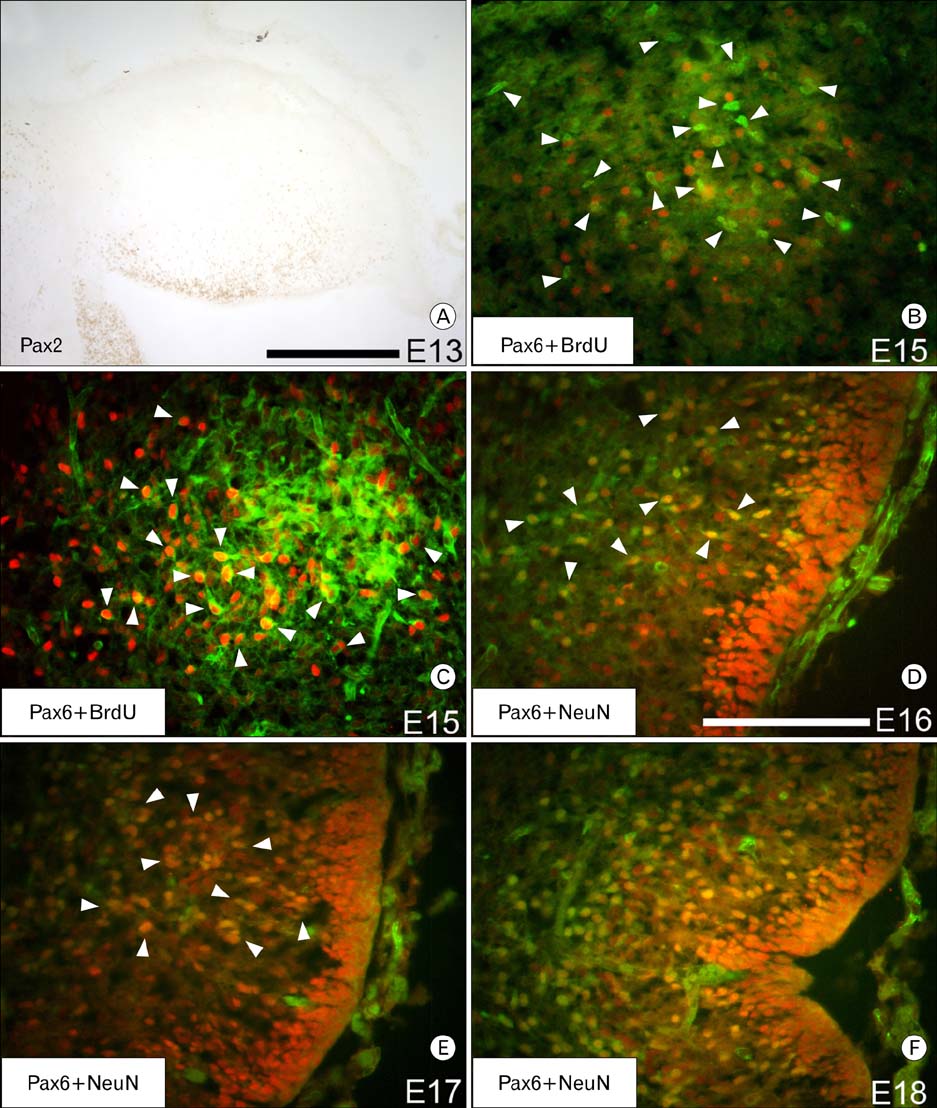
- Pax6, a paired homeobox DNA binding protein, has been found to be expressed in the cerebellum in both granule cells and their precursors in the external granular layer (EGL). In this study we have traced Pax6 expression through embryonic development in mice by using a polyclonal antibody against Pax6 and used it to study the cellular dispersal pattern of the EGL. During dispersal the EGL was thicker and Pax6 expression was more intense on the rostral side of the lateral corners of the cerebellum. Pax6 immunoreactive cells were found to be migrating from the EGL during the early stage of EGL dispersal, which suggested the early inward migration of granule cells. Double staining with various markers confirmed that the early-migrating cells are not Purkinje cells, interneurons or glia. Although the Pax6 immunoreactive cells within the cerebellum were not apparently proliferating, NeuN, a marker for postmitotic granule cells, was not expressed in these cells until E16. Furthermore, granule cells were observed migrating inwards from the EGL both during and after EGL dispersal. These early migrating granule cells populated the whole cerebellum. These findings offer novel views on specific stages of granule cell dispersal and migration.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A rare additional lobe of cerebellum, projecting from its superior surface
Satheesha Badagabettu Nayak, Suhani Sumalatha, Surekha Devadasa Shetty
Anat Cell Biol. 2022;55(3):376-379. doi: 10.5115/acb.22.048.
Reference
-
1. Altman J. Postnatal development of the cerebellar cortex in rat.I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972. 145:353–397.2. Altman J, Bayer SA. Embryonic development of the rat cerebellum. II. Translocation and regional distribution of the deep neurons. J Comp Neurol. 1985. 231:27–41.3. Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure, and functions. 1997. CRC Press;Chapter 9 and 10.4. Beierbach E. Lobulation in Mouse Cerebellum. 2001. University of Calgary, Canada: Masters thesis.5. Beimesche S, Neubauer A, Herzig S, et al. Tissue-specific transcriptional activity of a pancreatic islet cell-specific enhancer sequence/Pax6-binding site determined in normal adult tissues in vivo using transgenic mice. Mol Endocrinol. 1999. 13:718–728.6. Engelkamp D, Rashbass P, Seawright A, van Heyningen V. Role of Pax6 in development of the cerebellar system. Development. 1999. 126:3585–3596.7. Hanson I, Van Heyningen V. Pax6: more than meets the eye. Trends Genet. 1995. 11:268–272.8. Hanson IM, Seawright A, Hardman K, et al. Pax6 mutations in aniridia. Hum Mol Genet. 1993. 2:915–920.9. Hill RE, Favor J, Hogan BL, et al. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991. 354:522–525.10. Jensen P, Smeyne R, Goldowitz D. Analysis of cerebellar development in math1 null embryos and chimeras. J Neurosci. 2004. 24:2202–2211.11. Karam SD, Kim YS, Bothwell M. Granule cells migrate within raphes in the developing cerebellum: an evolutionarily conserved morphogenic event. J Comp Neurol. 2001. 440:127–135.12. Kawano H, Fukuda T, Kubo K, et al. Pax-6 is required for thalamocortical pathway formation in fetal rats. J Comp Neurol. 1999. 408:147–160.13. Luckner R, Obst-Pernberg K, Hirano S, Suzuki ST, Redies C. Granule cell raphes in the developing mouse cerebellum. Cell Tissue Res. 2001. 303:159–172.14. Mastick GS, Davis NM, Andrew GL, Easter SS Jr. Pax6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development. 1997. 124:1985–1997.15. Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999. 41:281–294.16. Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988. 457:44–52.17. Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003. 73:400–409.18. Yamasaki T, Kawaji K, Ono K, et al. Pax6 regulates granule cell polarization during parallel fiber formation in the developing cerebellum. Development. 2001. 128:3133–3144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maternal effect genes: Findings and effects on mouse embryo development
- The Effects of Narcotics on the Mouse Two-Cell Embryo Development
- Effect of Cumulus Cell Coculture on Early Mouse Embryonal Development in vitro
- Effectsof Protein and Amino Acid(Glutamate) on the Development of Late 2-Cell Mouse Embryo in vitro
- Effect of Human Oviduct Epithelial Cells and Vero Cell on Early Mouse Embryonal Development In Vitro