Cancer Res Treat.
2012 Jun;44(2):97-103.
Phase II Study of Consolidation Chemotherapy after Adjuvant or Primary Concurrent Chemoradiation Using Paclitaxel and Carboplatin to Treat High-Risk Early-Stage or Locally Advanced Cervical Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. kjwksh@snu.ac.kr
- 2Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Chung-Ang University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
This study investigated the efficacy and toxicity associated with consolidation chemotherapy using paclitaxel and carboplatin after concurrent chemoradiation (CCR) in cervical cancer patients.
MATERIALS AND METHODS
From a total of 37 patients, 19 with International Federation of Gynecology and Obstetrics (FIGO) stage IB1-IIA cervical cancer (group 1) underwent surgery followed by consolidation chemotherapy after CCR, and 18 with stage IIB-IVA disease (group 2) received consolidation chemotherapy after primary CCR. Three cycles of chemotherapy using paclitaxel (135 mg/m2) and carboplatin (AUC 5.0) were administered every 3 weeks for CCR therapy, and three cycles of consolidation chemotherapy using paclitaxel (175 mg/m2) and carboplatin (AUC 5.0) were used every 3 weeks after CCR.
RESULTS
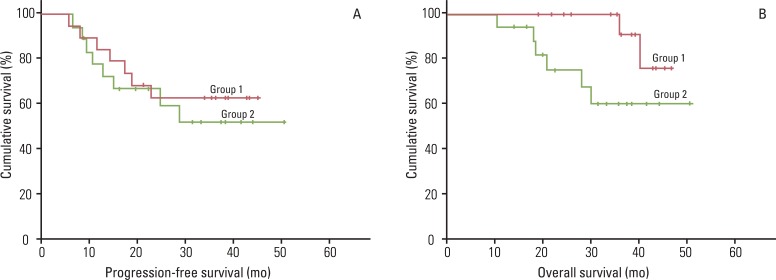
The complete and partial response rates were 77.8% and 22.2% in group 2. Moreover, the 3-year progression-free and overall survival rates were 62.7% and 90.9% in group 1, and 51.9% and 60% in group 2, respectively. The most common grade 3 or 4 hematologic toxicities observed were leukopenia (group 1, 10.5%; group 2, 13.0%) and neutropenia (group 1, 7.0%; group 2, 14.8%), and grade 3 or 4 diarrhea (group 1, 1.8%) and febrile illness (group 2, 1.9%) were the most frequently observed non-hematologic toxicities. When we compared these results with previous reports, consolidation chemotherapy after CCR using paclitaxel and carboplatin revealed a relatively lower complete response rate (77.8% vs. 87-100%, respectively) and shorter progression-free survival (51.9-62.7% vs. 81-86%, respectively) and overall survival (60-90.9% vs. 81-95%, respectively) in spite of similar toxicity findings.
CONCLUSION
Due to low efficacy results, consolidation chemotherapy using paclitaxel and carboplatin after CCR is not a feasible treatment regimen for high-risk early-stage or locally advanced cervical cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Suh DH, Kim JW, Kim K, Kang SB. Major clinical research advances in gynecologic cancer in 2010. J Gynecol Oncol. 2010; 21:209–218. PMID: 21278881.
Article2. Cervical cancer clinical practice guidelines in oncology (v.I.2011) [Internet]. National Comprehensive Cancer Network. cited 2010 Dec 24. Fort Washington, PA: National Comprehensive Cancer Network;Available from: http://www.Nccn.Org .3. Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001; 358:781–786. PMID: 11564482.
Article4. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999; 340:1144–1153. PMID: 10202165.
Article5. Petera J, Odrázka K, Frgala T, Spacek J. External beam radiotherapy and high-dose brachytherapy combined with cisplatin and paclitaxel in patients with advanced cervical carcinoma. Gynecol Oncol. 2005; 99:334–338. PMID: 16023181.
Article6. Kim YS, Shin SS, Nam JH, Kim YT, Kim YM, Kim JH, et al. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high-dose rate brachytherapy for locally advanced cervical cancer. Gynecol Oncol. 2008; 108:195–200. PMID: 17963825.
Article7. Vrdoljak E, Prskalo T, Omrcen T, Situm K, Boraska T, Frleta Ilić N, et al. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: results of a phase II study. Int J Radiat Oncol Biol Phys. 2005; 61:824–829. PMID: 15708262.
Article8. Chung YL, Jian JJ, Cheng SH, Hsieh CI, Tan TD, Chang HJ, et al. Extended-field radiotherapy and high-dose-rate brachytherapy with concurrent and adjuvant cisplatin-based chemotherapy for locally advanced cervical cancer: a phase I/II study. Gynecol Oncol. 2005; 97:126–135. PMID: 15790448.
Article9. Choi CH, Lee JW, Kim TJ, Kim WY, Nam HR, Kim BG, et al. Phase II study of consolidation chemotherapy after concurrent chemoradiation in cervical cancer: preliminary results. Int J Radiat Oncol Biol Phys. 2007; 68:817–822. PMID: 17379437.
Article10. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–3200. PMID: 12860964.
Article11. Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000; 92:699–708. PMID: 10793106.
Article12. Saito I, Kitagawa R, Fukuda H, Shibata T, Katsumata N, Konishi I, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB, persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505). Jpn J Clin Oncol. 2010; 40:90–93. PMID: 19825815.
Article13. Korean Gynecologic Oncology Group. A phase II trial of radiation therapy with concurrent paclitaxel/carboplatin chemotherapy in high-risk cervical cancer patients after radical hysterectomy [Internet]. ClinicalTrials.gov. cited 2012 Jan 18. Available from: http://clinicaltrialgov/ct2/show/NCT00340184?term=paclitaxel+and+carboplatin+and+concurrent+chemoradiation+and+cervical+cancer&rank=2 .14. Kim K, Chie EK, Wu HG, Ha SW, Kim JS, Kim IA, et al. Efficacy of paclitaxel and carboplatin as a regimen for postoperative concurrent chemoradiotherapy of high risk uterine cervix cancer. Gynecol Oncol. 2006; 101:398–402. PMID: 16330087.
Article15. Lee MY, Wu HG, Kim K, Ha SW, Kim JS, Kim IA, et al. Concurrent radiotherapy with paclitaxel/carboplatin chemotherapy as a definitive treatment for squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2007; 104:95–99. PMID: 16996117.
Article16. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.
Article17. de Vos FY, Bos AM, Gietema JA, Pras E, Van der Zee AG, de Vries EG, et al. Paclitaxel and carboplatin concurrent with radiotherapy for primary cervical cancer. Anticancer Res. 2004; 24:345–348. PMID: 15015619.18. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000; 18:1606–1613. PMID: 10764420.
Article19. Zhang MQ, Liu SP, Wang XE. Concurrent chemoradiotherapy with paclitaxel and nedaplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: preliminary results of a phase II study. Int J Radiat Oncol Biol Phys. 2010; 78:821–827. PMID: 20207507.
Article20. Wang X, Liu R, Ma B, Yang K, Tian J, Jiang L, et al. High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev. 2010; (7):CD007563. PMID: 20614461.
Article21. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009; 27:4649–4655. PMID: 19720909.
Article22. Kitagawa R, Katsumata N, Yamanaka Y, Ando M, Fujiwara Y, Kasamatsu T. Phase II trial of paclitaxel and carboplatin in patients with recurrent or metastatic cervical carcinoma. J Clin Oncol. 2004; 22(14S):5048.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent Chemoradiation with Weekly Paclitaxel in Locally Advanced Non-small Cell Lung Cancer
- Efficacy and Safety of Concurrent Chemoradiotherapy of Paclitaxel and Carboplatin as Adjuvant Therapy after Primary Surgery in High-risk Cervical Cancer
- Efficacy of weekly paclitaxel and concurrent radiation therapy for locally advanced non-small cell lung cancer
- Recent Management of FIGO stage IB2 Cervical Cancer
- Adjuvant therapy in cervical cancer patients with high risk factors