Cancer Res Treat.
2012 Mar;44(1):1-10.
Present Status and Problems on Molecular Targeted Therapy of Cancer
- Affiliations
-
- 1Division of Medical Oncology, Kinki University School of Medicine, Osaka, Japan. nsaijo@an.em-net.ne.jp
Abstract
- Numerous clinical trials of molecular targeted drugs for cancer have been conducted, with remarkable results for certain drugs and accumulation of "negative data" causing a hitch in the development plan for some other compounds. Five recent issues and problems of molecular targeted therapies were discussed critically. Drug discovery and effects against driver mutations (activating mutations) and problems: possibility for circumventing inherent and acquired resistance with the aim of achieving radical cure. Synthetic lethality: reasonable patient selection in individualized treatment strategy. Response rate and progression-free survival improvement with or without overall survival benefit and enhancement of toxicity in bevacizumab therapy: best endpoints for the evaluation of effect of antiangiogenic therapy. Negative data on small-molecule targeted therapy, primarily vascular endothelial growth factor tyrosine kinase inhibitors: loose GO or NO-GO decision criteria for further development of new compounds in early clinical trials. Effect of immunotherapy: difficulty to verify by proof of principle study. We are faced to many questions for the development of efficient personalized therapy. Accumulation of scientific global preclinical and clinical evidences is essential to use these new therapeutic modalities for the improvement of oncologic health care.
Keyword
MeSH Terms
-
Bevacizumab
Antibodies, Monoclonal, Humanized
Delivery of Health Care
Disease-Free Survival
Drug Discovery
Endpoint Determination
Humans
Molecular Targeted Therapy
Patient Selection
Protein-Tyrosine Kinases
Social Change
Vascular Endothelial Growth Factor A
Antibodies, Monoclonal, Humanized
Protein-Tyrosine Kinases
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Weinstein IB, Joe AK. Mechanisms of disease: oncogene addiction-a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006; 3:448–457. PMID: 16894390.
Article2. Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Jänne PA, Riely GJ, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009; 15:5267–5273. PMID: 19671843.
Article3. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363:1693–1703. PMID: 20979469.4. Chapman PB, Hauschild A, Robert C, Larkin JM, Haanen JB, Ribas A, et al. Phase III randomized open label, multicenter trial (BRIM3) comparing BRAF inhibitor vemurafenib with dacarbazine (DTIC) in patients with V600EBRAF-mutated melanoma. J Clin Oncol. 2011; 29(S):LBA4.5. CTV News: Vemurafenib in melanoma: was its phase 3 trial unethical? [Internet]. cited 2011 Dec 20. Scarborough, ON: CTV Television Network;Available from: http://healthblog.ctv.ca .6. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500. PMID: 15118125.7. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139. PMID: 15118073.
Article8. Siehl J, Thiel E. C-kit, GIST, and imatinib. Recent Results Cancer Res. 2007; 176:145–151. PMID: 17607922.
Article9. Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007; 98:1817–1824. PMID: 17888036.
Article10. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809–819. PMID: 20818844.
Article11. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007; 448:561–566. PMID: 17625570.
Article12. Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011; 29:3574–3579. PMID: 21825258.13. Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010; 363:1734–1739. PMID: 20979473.
Article14. Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011; 71:6051–6060. PMID: 21791641.
Article15. Saijo N. Critical comments for roles of biomarkers in the diagnosis and treatment of cancer. Cancer Treat Rev. 2012; 38:63–67. PMID: 21652149.
Article16. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011; 3:75ra26.
Article17. Rehman FL, Lord CJ, Ashworth A. Synthetic lethal approaches to breast cancer therapy. Nat Rev Clin Oncol. 2010; 7:718–724. PMID: 20956981.
Article18. Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005; 434:917–921. PMID: 15829967.
Article19. Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005; 434:913–917. PMID: 15829966.
Article20. McCabe N, Lord CJ, Tutt AN, Martin NM, Smith GC, Ashworth A. BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of Poly (ADP-Ribose) polymerase: an issue of potency. Cancer Biol Ther. 2005; 4:934–936. PMID: 16251802.
Article21. McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006; 66:8109–8115. PMID: 16912188.
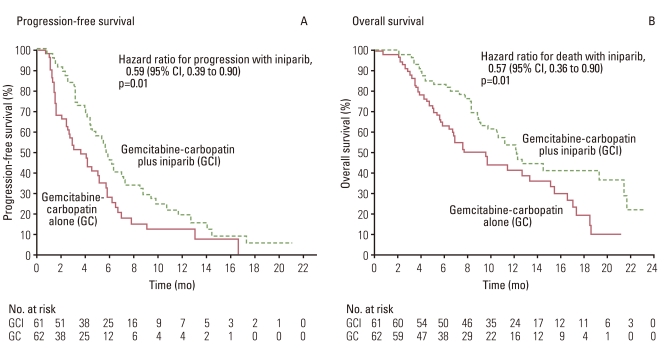
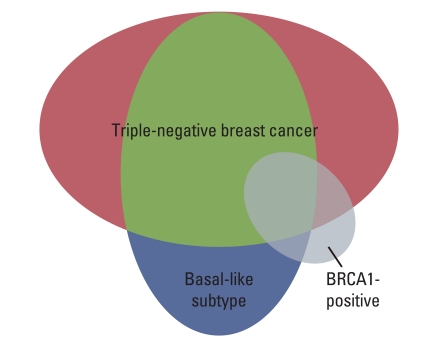
Article22. Ledermann JA, Harter P, Gourley C, Friedlnder M, Vergote IB, Rustin GJ, et al. Phase II randomized placebo-controlled study of olaparib (AZD2281) in patients with platinum-sensitive relapsed serious ovarian cancer (PSR SOC). J Clin Oncol. 2011; 29(S):5003.23. O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011; 364:205–214. PMID: 21208101.24. O'Shaughnessy J, Schwartzberg LS, Danso MA, Rugo HS, Miler K, Yardly DA, et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC). J Clin Oncol. 2011; 29(S):1007. PMID: 21205758.25. Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011; 125:627–636. PMID: 21161370.
Article26. Saijo N. Problems involved in the clinical trials for non-small cell lung carcinoma. Cancer Treat Rev. 2012; 2011 Jul 19 [Epub]. http://dx.doi.org/10.1016/j.ctrv.2011.06.001 .
Article27. Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005; 23:792–799. PMID: 15681523.
Article28. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007; 357:2666–2676. PMID: 18160686.
Article29. Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010; 28:3239–3247. PMID: 20498403.
Article30. Robert NJ, Diéras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011; 29:1252–1260. PMID: 21383283.
Article31. Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005; 352:476–487. PMID: 15689586.
Article32. Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005; 23:3502–3508. PMID: 15908660.
Article33. Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26:2013–2019. PMID: 18421054.
Article34. Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Colangelo LH, Lopa SH, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009; 27:3385–3390. PMID: 19414665.
Article35. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006; 355:2542–2550. PMID: 17167137.
Article36. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009; 27:1227–1234. PMID: 19188680.
Article37. Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007; 370:2103–2111. PMID: 18156031.
Article38. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011; 29:3968–3976. PMID: 21844504.
Article39. Burger RA, Brady MF, Bookman MA, Walker JL, Homesley HD, Fowker J, et al. Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): a Gynecologic Oncology Group study. J Clin Oncol. 2010; 28(18S):LBA1.
Article40. Kristensen G, Perren T, Qian W, Pfisterer J, Ledermann JA, Joly F, et al. Result of interim analysis of overall survival in the GCIG ICON7 phase III randomized trial of bevacizumab in women with newly diagnosed ovarian cancer. J Clin Oncol. 2011; 29(S):LBA5006.
Article41. Aghajanian C, Finkler NJ, Ruterford T, Smith DA, Yi J, Parmar H, et al. Oceans: a randomized, double-blinded, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoncal, or fallopian tube cancer. J Clin Oncol. 2011; 29(S):LBA5007.42. Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009; 27:4966–4972. PMID: 19720913.
Article43. Burstein HJ. Bevacizumab for advanced breast cancer: all tied up with a RIBBON? J Clin Oncol. 2011; 29:1232–1235. PMID: 21383305.
Article44. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353:123–132. PMID: 16014882.
Article45. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957. PMID: 19692680.
Article46. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008; 372:1809–1818. PMID: 19027483.
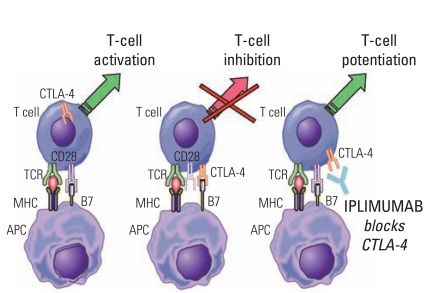
Article47. Schiller JH, Akerley WL, Brugger W, Ferrari D, Garmey EG, Gerber DE, et al. Results of ARQ 197-209: a global randomized placebo-controlled phase II clinical trial of erlotinib plus ARQ 197 versus erlotinib plus placebo in previously treated EGFR inhibitor-naïve patients with locally advanced metastatic non-small cell lung cancer (NSCLC). J Clin Oncol. 2010; 28(18S):LBA7502.48. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010; 363:411–422. PMID: 20818862.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Systemic Therapy for Advanced Hepatocellular Carcinoma: Targeted Therapy and Immunotherapy
- New Targeted Therapy for Non-Small Cell Lung Cancer
- Molecular Targeted Therapy in Cancer
- Molecular targeted therapy of thyroid cancer