Cancer Res Treat.
2011 Jun;43(2):124-130.
Up-regulation of the DR5 Expression by Proteasome Inhibitor MG132 Augments TRAIL-Induced Apoptosis in Soft Tissue Sarcoma Cell Lines
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine and Institute for Clinical Molecular Biology Research, Soonchunhyang University College of Medicine, Seoul, Korea.
- 2Catholic Research Institutes of Medical Science, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 3Division of Oncology, Department of Internal Medicine, Yeouido St. Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 4Division of Oncology, Department of Internal Medicine, Incheon St. Mary's Hospital, The Catholic University of Korea School of Medicine, Incheon, Korea. jhbyun37@catholic.ac.kr
Abstract
- PURPOSE
Current chemotherapeutics for treating locally advanced or metastatic soft tissue sarcomas (STS) are limited. Accordingly, the present in vitro study was conducted to evaluate the effects of treatment of STS cells with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) applied as a single agent or in combination with a proteasome inhibitor, MG132.
MATERIALS AND METHODS
Sensitivity to TRAIL and activity of TRAIL-induced apoptotic pathways were analyzed in four STS cell lines: HTB-82 (rhabdomyosarcoma), HT-1080 (fibrosarcoma), HTB-93 (synovial sarcoma), and HTB-94 (chondrosarcoma). Reduction of the dye dimethylthiazolyl 2,5 diphenyltetrazolium bromide (MTT) was used to evaluate cytotoxic activity; western blots were used to evaluate TRAIL-induced apoptosis.
RESULTS
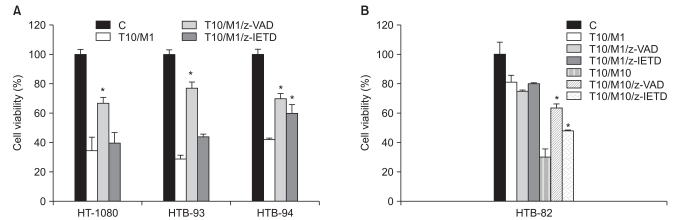
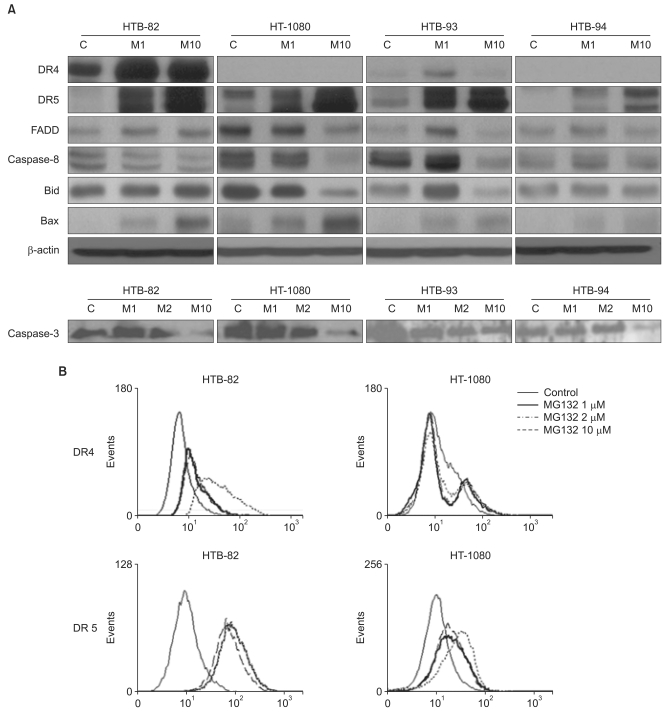
TRAIL induced apoptosis in HTB-93 cells, but had little effect in HTB-82, HT-1080, or HTB-94 cells. Expression of TRAIL receptor-1 and -2 did not correlate with sensitivity to TRAIL. Co-incubation of cells with TRAIL and a proteasome inhibitor, MG132, augmented the apoptotic effect of TRAIL in both TRAIL-sensitive and TRAIL-resistant cells. This effect was due to up-regulation of TRAIL receptors and members of the pro-apoptotic BCL-2 family by MG132.
CONCLUSION
These data show that combining TRAIL with MG132 enhances apoptosis and overcomes TRAIL resistance. This restoration of TRAIL sensitivity occurs through an increase in the expression of death receptor 5 and of pro-apoptotic BCL-2 family members such as BAX.
MeSH Terms
-
Apoptosis
Blotting, Western
Cell Line
Humans
Leupeptins
Necrosis
Proteasome Endopeptidase Complex
Proteasome Inhibitors
Receptors, TNF-Related Apoptosis-Inducing Ligand
Sarcoma
TNF-Related Apoptosis-Inducing Ligand
Up-Regulation
Leupeptins
Proteasome Endopeptidase Complex
Proteasome Inhibitors
Receptors, TNF-Related Apoptosis-Inducing Ligand
TNF-Related Apoptosis-Inducing Ligand
Figure
Reference
-
1. Clarkson P, Ferguson PC. Management of soft tissue sarcomas of the extremities. Expert Rev Anticancer Ther. 2004; 4:237–246. PMID: 15056054.
Article2. Antman K, Crowley J, Balcerzak SP, Rivkin SE, Weiss GR, Elias A, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993; 11:1276–1285. PMID: 8315425.
Article3. Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996; 271:12687–12690. PMID: 8663110.
Article4. Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997; 276:111–113. PMID: 9082980.
Article5. Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997; 16:5386–5397. PMID: 9311998.
Article6. Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, et al. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997; 186:1165–1170. PMID: 9314565.
Article7. Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997; 7:1003–1006. PMID: 9382840.
Article8. Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998; 273:14363–14367. PMID: 9603945.
Article9. Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005; 4:139–163. PMID: 15725726.
Article10. Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002; 2:420–430. PMID: 12189384.
Article11. Hao C, Beguinot F, Condorelli G, Trencia A, Van Meir EG, Yong VW, et al. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apotosis in human malignant glioma cells. Cancer Res. 2001; 61:1162–1170. PMID: 11221847.12. Pawlowski JE, Nesterov A, Scheinman RI, Johnson TR, Kraft AS. NF-kappa B does not modulate sensitivity of renal carcinoma cells to TNF alpha-related apoptosis-inducing ligand (TRAIL). Anticancer Res. 2000; 20:4243–4255. PMID: 11205254.13. Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004; 4:349–360. PMID: 15122206.
Article14. Khanbolooki S, Nawrocki ST, Arumugam T, Andtbacka R, Pino MS, Kurzrock R, et al. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006; 5:2251–2260. PMID: 16985059.15. Johnson TR, Stone K, Nikrad M, Yeh T, Zong WX, Thompson CB, et al. The proteasome inhibitor PS-341 overcomes TRAIL resistance in Bax and caspase 9-negative or Bcl-xL overexpressing cells. Oncogene. 2003; 22:4953–4963. PMID: 12902978.
Article16. Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998; 94:491–501. PMID: 9727492.
Article17. Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998; 94:481–490. PMID: 9727491.
Article18. Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992; 10:52–60. PMID: 1727926.
Article19. Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993; 364:806–809. PMID: 7689176.
Article20. Sayers TJ, Murphy WJ. Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy. Cancer Immunol Immunother. 2006; 55:76–84. PMID: 15864587.
Article21. Voortman J, Resende TP, Abou El, Giaccone G, Kruyt FA. TRAIL therapy in non-small cell lung cancer cells: sensitization to death receptor-mediated apoptosis by proteasome inhibitor bortezomib. Mol Cancer Ther. 2007; 6:2103–2112. PMID: 17620439.
Article22. Chen JJ, Chou CW, Chang YF, Chen CC. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. J Immunol. 2008; 180:8030–8039. PMID: 18523266.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Proteasome Inhibitor MG132 Sensitizes Lung Cancer Cells to TRAIL-induced Apoptosis by Inhibiting NF-kappaB Activation
- The Mechanism of Proteasome Inhibitor-Induced Apoptosis in Lung Cancer Cells
- Human hepatocellular carcinoma cells resist to TRAIL-induced apoptosis, and the resistance is abolished by cisplatin
- MDL-12330A potentiates TRAIL-induced apoptosis in gastric cancer cells through CHOP-mediated DR5 upregulation
- Hydrogen Peroxide Upregulates TNF-Related Apoptosis-Inducing Ligand (TRAIL) Expression in Human Astroglial Cells, and Augments Apoptosis of T Cells