Cancer Res Treat.
2011 Jun;43(2):89-95.
Implications of Bone-Only Metastases in Breast Cancer: Favorable Preference with Excellent Outcomes of Hormone Receptor Positive Breast Cancer
- Affiliations
-
- 1Division of Hematology/Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. imyh00@skku.edu
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
The aim of the current study was to determine the incidence, clinical presentation, and treatment outcomes of "bone-only metastases" in patients with breast cancer and to analyze the impact of hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status on prognosis.
MATERIALS AND METHODS
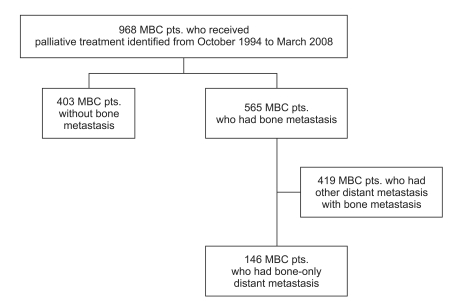
Between 1994 and 2007, of 968 patients with metastatic breast cancer who underwent palliative management at Samsung Medical Center, 565 (57%) relapsed with distant metastases. Of the 968, 146 (15%) had bone-only metastases during a median follow-up period of 75 months. Among the 146 patients with bone-only metastases, 122 (84%) were relapsed patients after curative surgery and 24 (26%) were initially metastatic cases.
RESULTS
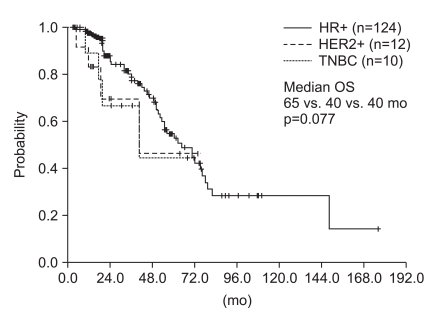
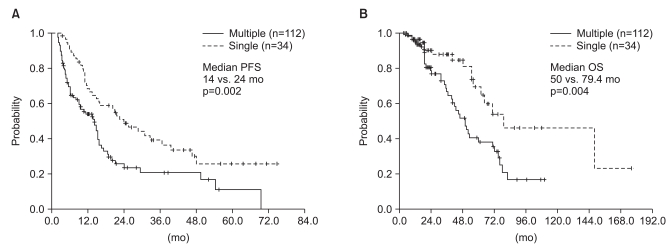
The median time from primary surgery to bone-only metastases of the 122 patients was 37 months (95% confidence interval [CI], 27 to 46 months). Bone-only metastases were more common in the HR-positive group than in the other subtypes (85% for HR+; 8.2% for HER2+; 6.8% for triple negative. Among all 146 patients, 75 (51%) were treated with hormone therapy. The median post-relapse progression-free survival was 15 months (95%CI, 13 to 17 months). The median overall survival was much longer in the HR+ patients than the HER2+ and triple negative breast cancer patients with marginal statistical significance (65 vs. 40 vs. 40 months, p=0.077).
CONCLUSION
Breast cancer patients with "bone-only metastases" had excellent clinical outcomes. Further study is now warranted to reveal the underlying biology that regulates the behavior of this indolent tumor, as it should identify 'favorable tumor characteristics' in addition to 'favorable preferential metastatic site.'
Keyword
MeSH Terms
Figure
Reference
-
1. Coleman RE. Skeletal complications of malignancy. Cancer. 1997; 80(8 Suppl):1588–1594. PMID: 9362426.
Article2. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987; 55:61–66. PMID: 3814476.
Article3. Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999; 49:33–64. 1PMID: 10200776.
Article4. Sherry MM, Greco FA, Johnson DH, Hainsworth JD. Breast cancer with skeletal metastases at initial diagnosis. Distinctive clinical characteristics and favorable prognosis. Cancer. 1986; 58:178–182. PMID: 2423224.
Article5. Sherry MM, Greco FA, Johnson DH, Hainsworth JD. Metastatic breast cancer confined to the skeletal system: an indolent disease. Am J Med. 1986; 81:381–386. PMID: 2428242.6. Lee YT. Patterns of metastasis and natural courses of breast carcinoma. Cancer Metastasis Rev. 1985; 4:153–172. PMID: 3893684.7. Kamby C, Ejlertsen B, Andersen J, Birkler NE, Rytter L, Zedeler K, et al. The pattern of metastases in human breast cancer: influence of systemic adjuvant therapy and impact on survival. Acta Oncol. 1988; 27:715–719. PMID: 3219223.
Article8. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998; 77:336–340. PMID: 9461007.
Article9. Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, et al. Breast cancer: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009; 7:122–192. PMID: 19200416.
Article10. Nguyen DX, Massagué J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007; 8:341–352. PMID: 17440531.
Article11. Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008; 68:3108–3114. PMID: 18451135.
Article12. Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008; 359:2814–2823. PMID: 19109576.
Article13. Lacroix M. Significance, detection and markers of disseminated breast cancer cells. Endocr Relat Cancer. 2006; 13:1033–1067. PMID: 17158753.
Article14. Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889; 133:571–573.
Article15. Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst. 1999; 91:80–85. PMID: 9890174.
Article16. Pfitzenmaier J, Ellis WJ, Arfman EW, Hawley S, McLaughlin PO, Lange PH, et al. Telomerase activity in disseminated prostate cancer cells. BJU Int. 2006; 97:1309–1313. PMID: 16686730.
Article17. Weckermann D, Müller P, Wawroschek F, Harzmann R, Riethmüller G, Schlimok G. Disseminated cytokeratin positive tumor cells in the bone marrow of patients with prostate cancer: detection and prognostic value. J Urol. 2001; 166:699–703. PMID: 11458120.
Article18. Demicheli R. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin Cancer Biol. 2001; 11:297–306. PMID: 11513565.
Article19. Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009; 16:67–78. PMID: 19573813.
Article20. Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A. 2003; 100:7737–7742. PMID: 12808139.
Article21. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–1717. PMID: 15894097.