Cancer Res Treat.
2010 Mar;42(1):12-17.
Efficacy and Safety of Docetaxel Plus Prednisolone Chemotherapy for Metastatic Hormone-Refractory Prostate Adenocarcinoma: Single Institutional Study in Korea
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jaelyun@amc.seoul.kr
- 2Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
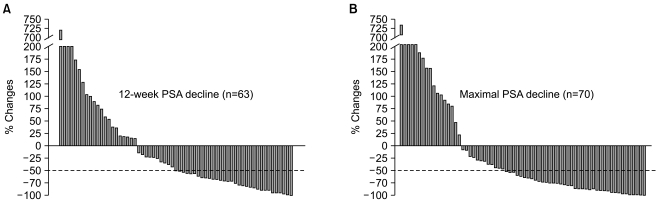
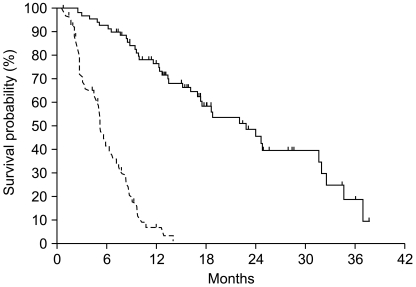
To assess the efficacy and safety of treating Korean patients with metastatic hormone-refractory prostate cancer (HRPC) using docetaxel plus prednisolone chemotherapy. MATERIALS AND METHODS: This was a retrospective cohort study performed in 98 patients with metastatic HRPC between October 2003 and April 2008. After screening, 72 patients fit the eligibility criteria for inclusion in this study. Treatment consisted of 5 mg prednisolone twice daily and 75 mg/m2 docetaxel once every 3 weeks. RESULTS: Patient demographic characteristics included: median age 67 years (range, 51~86), median ECOG performance status 1 (0~2), Gleason score > or =8 in 61 patients (86%), and median serum PSA 45.5 ng/mL (range, 3.7~2,420.0). A total of 405 cycles of treatment were administered with a median 6 cycles (range, 1~20) per patient. The median docetaxel dose-intensity was 24.4 mg/m2/week (range, 17.5~25.6). A PSA response was seen in 51% of 63 evaluable patients at 12 weeks and maximal PSA decline > or =50% in 59% of 70 evaluable patients. Tumor response was evaluated in 13 patients, 4 patients achieved PR, and 5 patients had SD with a response rate of 31%. With a median follow-up duration of 23.1 months (95%CI, 16.7~29.5), the median time to PSA progression was 5.1 months (95%CI, 4.5~5.8) and median overall survival was 22.8 months (95%CI, 16.6~29.1). Nine (13%) patients experienced grade 3 or higher febrile neutropenia. CONCLUSION: This chemotherapy regimen (docetaxel every 3 weeks plus prednisolone daily) demonstrated a strong response in Korean patients with metastatic HRPC, while the toxicity profile was manageable and similar to that observed in Western patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96. PMID: 18287387.
Article2. Ministry for Health, Welfare and Family Affairs. Annual report of cancer incidence (2005) and survival (1993-2005) in Korea. 2008.3. Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002; 52:154–179. PMID: 12018929.
Article4. Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996; 14:1756–1764. PMID: 8656243.
Article5. Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, Hars V, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the Cancer and Leukemia Group B 9182 study. J Clin Oncol. 1999; 17:2506–2513. PMID: 10561316.
Article6. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004; 351:1513–1520. PMID: 15470214.
Article7. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351:1502–1512. PMID: 15470213.
Article8. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26:1148–1159. PMID: 18309951.
Article9. de Wit R. Chemotherapy in hormone-refractory prostate cancer. BJU Int. 2008; 101(Suppl 2):11–15. PMID: 18307687.
Article10. Naito S, Tsukamoto T, Koga H, Harabayashi T, Sumiyoshi Y, Hoshi S, et al. Docetaxel plus prednisolone for the treatment of metastatic hormone-refractory prostate cancer: a multicenter Phase II trial in Japan. Jpn J Clin Oncol. 2008; 38:365–372. PMID: 18417502.
Article11. Joung JY, Jeong IG, Han KS, Kim TS, Yang SO, Seo HK, et al. Docetaxel chemotherapy of Korean patients with hormone-refractory prostate cancer: comparative analysis between 1st-line and 2nd-line docetaxel. Yonsei Med J. 2008; 49:775–782. PMID: 18972598.12. Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002; 20:3683–3690. PMID: 12202670.
Article13. Millward MJ, Boyer MJ, Lehnert M, Clarke S, Rischin D, Goh BC, et al. Docetaxel and carboplatin is an active regimen in advanced non-small-cell lung cancer: a phase II study in Caucasian and Asian patients. Ann Oncol. 2003; 14:449–454. PMID: 12598352.14. Rathkopf D, Carducci MA, Morris MJ, Slovin SF, Eisenberger MA, Pili R, et al. Phase II trial of docetaxel with rapid androgen cycling for progressive noncastrate prostate cancer. J Clin Oncol. 2008; 26:2959–2965. PMID: 18565882.
Article15. Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet. 1999; 36:99–114. PMID: 10092957.
Article16. Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008; 26:242–245. PMID: 18182665.
Article17. Wailoo A, Sutton A, Morgan A. The risk of febrile neutropenia in patients with non-small-cell lung cancer treated with docetaxel: a systematic review and meta-analysis. Br J Cancer. 2009; 100:436–441. PMID: 19190633.
Article18. Lee JL, Ryu MH, Chang HM, Kim TW, Yook JH, Oh ST, et al. A phase II study of docetaxel as salvage chemotherapy in advanced gastric cancer after failure of fluoropyrimidine and platinum combination chemotherapy. Cancer Chemother Pharmacol. 2008; 61:631–637. PMID: 17520252.
Article19. Klastersky J, Awada A, Aoun M, Paesmans M. Should the indications for the use of myeloid growth factors for the prevention of febrile neutropenia in cancer patients be extended? Curr Opin Oncol. 2009; 21:297–302. PMID: 19509500.
Article20. Pascoe J, Steven N. Antibiotics for the prevention of febrile neutropenia. Curr Opin Hematol. 2009; 16:48–52. PMID: 19057204.
Article21. Di Lorenzo G, Autorino R, Figg WD, De Placido S. Hormone-refractory prostate cancer: where are we going? Drugs. 2007; 67:1109–1124. PMID: 17521214.22. Attard G, Reid AH, A'Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009; 27:3742–3748. PMID: 19470933.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Comparison of Ketoconazole-Prednisolone Combination Therapy with Prednisolone Alone in Patients with Hormone Refractory Prostate Cancer
- What's New in Hormone-refractory Prostate Cancer Treatment
- Ketoconazole with Prednisolone for the Treatment of Hormone Refractory Prostate Cancer
- Docetaxel Chemotherapy of Korean Patients with Hormone-refractory Prostate Cancer: Comparative Analysis between 1st-line and 2nd-line Docetaxel