Clin Endosc.
2013 Nov;46(6):603-610.
Endoscopic Molecular Imaging: Status and Future Perspective
- Affiliations
-
- 1Department of Gastroenterology and Oncology, Institute of Health Biosciences, The University of Tokushima Graduate School, Tokushima, Japan. muguruma.clin.med@gmail.com
Abstract
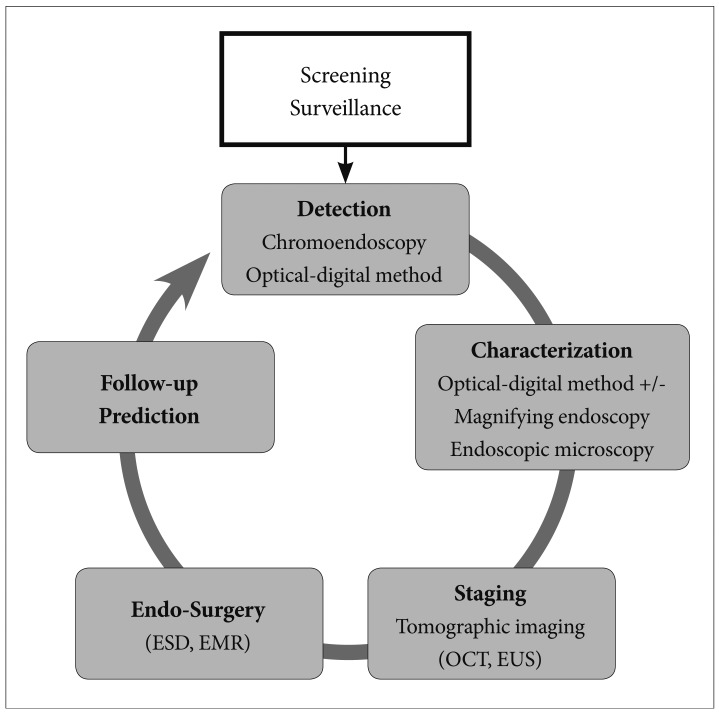
- During the last decade, researchers have made great progress in the development of new image processing technologies for gastrointestinal endoscopy. However, diagnosis using conventional endoscopy with white light optical imaging is essentially limited, and ultimately, we still rely on the histopathological diagnosis from biopsy specimens. Molecular imaging represents the most novel imaging methods in medicine, and the future of endoscopic diagnosis is likely to be impacted by a combination of biomarkers and technology. Endoscopic molecular imaging can be defined as the visualization of molecular characteristics with endoscopy. These innovations will allow us not only to locate a tumor or dysplastic lesion but also to visualize its molecular characteristics and the activity of specific molecules and biological processes that affect tumor behavior and/or its response to therapy. In the near future, these promising technologies will play a central role in endoluminal oncology.
MeSH Terms
Figure
Reference
-
1. Sivak MV. Gastrointestinal endoscopy: past and future. Gut. 2006; 55:1061–1064. PMID: 16849338.
Article2. Cotton PB, Barkun A, Ginsberg G, et al. Diagnostic endoscopy: 2020 vision. Gastrointest Endosc. 2006; 64:395–398. PMID: 16923489.
Article3. Uedo N, Iishi H, Tatsuta M, et al. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest Endosc. 2005; 62:521–528. PMID: 16185965.
Article4. Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004; 9:568–577. PMID: 15189095.
Article5. Jung SW, Lim KS, Lim JU, et al. Flexible spectral imaging color enhancement (FICE) is useful to discriminate among non-neoplastic lesion, adenoma, and cancer of stomach. Dig Dis Sci. 2011; 56:2879–2886. PMID: 21800158.
Article6. Hong SN, Choe WH, Lee JH, et al. Prospective, randomized, back-to-back trial evaluating the usefulness of i-SCAN in screening colonoscopy. Gastrointest Endosc. 2012; 75:1011–1021. PMID: 22381530.
Article7. Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004; 127:706–713. PMID: 15362025.
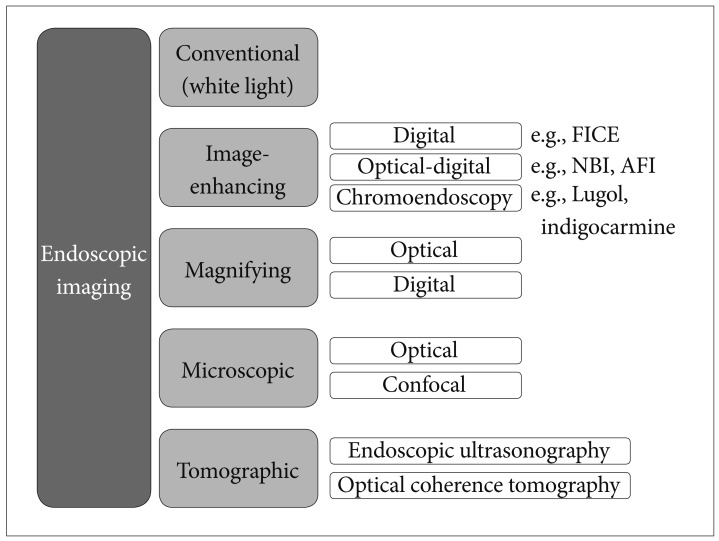
Article8. Tajiri H, Niwa H. Proposal for a consensus terminology in endoscopy: how should different endoscopic imaging techniques be grouped and defined? Endoscopy. 2008; 40:775–778. PMID: 18698532.
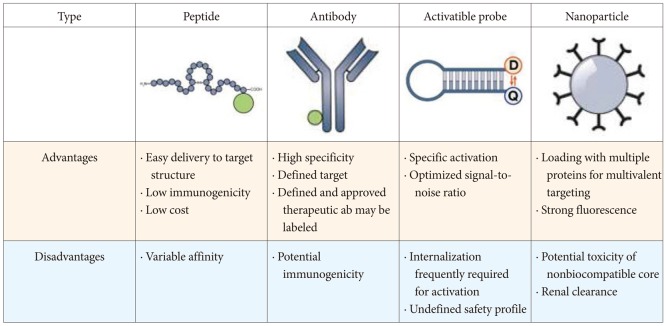
Article9. Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001; 219:316–333. PMID: 11323453.
Article10. Thakur M, Lentle BC. Report of a summit on molecular imaging. Radiology. 2005; 236:753–755. PMID: 16118158.
Article11. Mahmood U, Wallace MB. Molecular imaging in gastrointestinal disease. Gastroenterology. 2007; 132:11–14. PMID: 17241854.
Article12. Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998; 339:1277–1284. PMID: 9791143.
Article13. Keller R, Winde G, Eisenhawer C, et al. Immunoscopy: a technique combining endoscopy and immunofluorescence for diagnosis of colorectal carcinoma. Gastrointest Endosc. 1998; 47:154–161. PMID: 9512281.14. Pasricha PJ, Motamedi M. Optical biopsies, "bioendoscopy," and why the sky is blue: the coming revolution in gastrointestinal imaging. Gastroenterology. 2002; 122:571–575. PMID: 11832471.
Article15. Fujimoto JG, Brezinski ME, Tearney GJ, et al. Optical biopsy and imaging using optical coherence tomography. Nat Med. 1995; 1:970–972. PMID: 7585229.
Article16. Weissleder R. Molecular imaging in cancer. Science. 2006; 312:1168–1171. PMID: 16728630.
Article17. Goetz M, Hoetker MS, Diken M, Galle PR, Kiesslich R. In vivo molecular imaging with cetuximab, an anti-EGFR antibody, for prediction of response in xenograft models of human colorectal cancer. Endoscopy. 2013; 45:469–477. PMID: 23580409.
Article18. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011; 17:1685–1691. PMID: 22057348.
Article19. Keller R, Winde G, Terpe HJ, Foerster EC, Domschke W. Fluorescence endoscopy using a fluorescein-labeled monoclonal antibody against carcinoembryonic antigen in patients with colorectal carcinoma and adenoma. Endoscopy. 2002; 34:801–807. PMID: 12244502.
Article20. Marten K, Bremer C, Khazaie K, et al. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology. 2002; 122:406–414. PMID: 11832455.
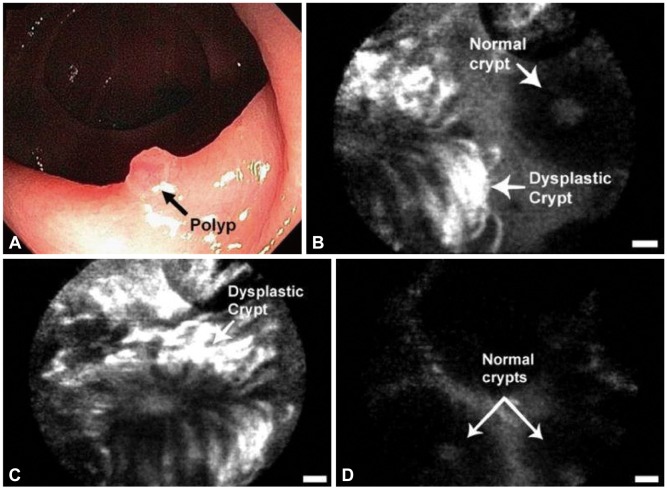
Article21. Hsiung PL, Hardy J, Friedland S, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008; 14:454–458. PMID: 18345013.
Article22. Ito S, Muguruma N, Kusaka Y, et al. Detection of human gastric cancer in resected specimens using a novel infrared fluorescent anti-human carcinoembryonic antigen antibody with an infrared fluorescence endoscope in vitro. Endoscopy. 2001; 33:849–853. PMID: 11571680.
Article23. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013; 339:1546–1558. PMID: 23539594.
Article24. Funovics MA, Alencar H, Montet X, Weissleder R, Mahmood U. Simultaneous fluorescence imaging of protease expression and vascularity during murine colonoscopy for colonic lesion characterization. Gastrointest Endosc. 2006; 64:589–597. PMID: 16996355.25. Petrovsky A, Schellenberger E, Josephson L, Weissleder R, Bogdanov A Jr. Near-infrared fluorescent imaging of tumor apoptosis. Cancer Res. 2003; 63:1936–1942. PMID: 12702586.26. Citrin D, Lee AK, Scott T, et al. In vivo tumor imaging in mice with near-infrared labeled endostatin. Mol Cancer Ther. 2004; 3:481–488. PMID: 15078992.27. Hoetker MS, Kiesslich R, Diken M, et al. Molecular in vivo imaging of gastric cancer in a human-murine xenograft model: targeting epidermal growth factor receptor. Gastrointest Endosc. 2012; 76:612–620. PMID: 22771099.
Article28. Goetz M, Ziebart A, Foersch S, et al. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology. 2010; 138:435–446. PMID: 19852961.
Article29. Foersch S, Kiesslich R, Waldner MJ, et al. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut. 2010; 59:1046–1055. PMID: 20639250.
Article30. Bando T, Muguruma N, Ito S, et al. Basic studies on a labeled anti-mucin antibody detectable by infrared-fluorescence endoscopy. J Gastroenterol. 2002; 37:260–269. PMID: 11993509.
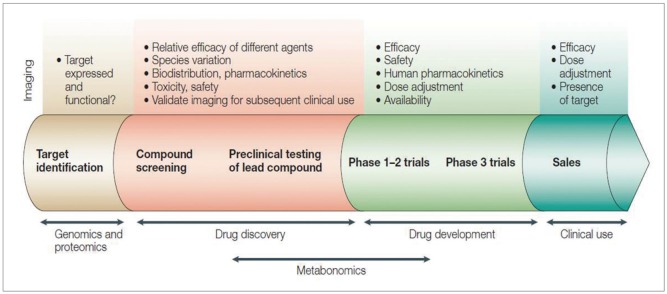
Article31. Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov. 2003; 2:123–131. PMID: 12563303.
Article32. Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology. 2010; 138:828–833. PMID: 20096697.
Article33. Kelly K, Alencar H, Funovics M, Mahmood U, Weissleder R. Detection of invasive colon cancer using a novel, targeted, library-derived fluorescent peptide. Cancer Res. 2004; 64:6247–6251. PMID: 15342411.
Article34. Fujikawa Y, Urano Y, Komatsu T, et al. Design and synthesis of highly sensitive fluorogenic substrates for glutathione S-transferase and application for activity imaging in living cells. J Am Chem Soc. 2008; 130:14533–14543. PMID: 18841967.
Article35. Joshi BP, Liu Z, Elahi SF, Appelman HD, Wang TD. Near-infrared-labeled peptide multimer functions as phage mimic for high affinity, specific targeting of colonic adenomas in vivo (with videos). Gastrointest Endosc. 2012; 76:1197–1206. PMID: 23022051.
Article36. Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003; 21:41–46. PMID: 12459735.
Article37. Mordon S, Devoisselle JM, Soulie-Begu S, Desmettre T. Indocyanine green: physicochemical factors affecting its fluorescence in vivo. Microvasc Res. 1998; 55:146–152. PMID: 9521889.38. Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009; 69:1268–1272. PMID: 19176373.
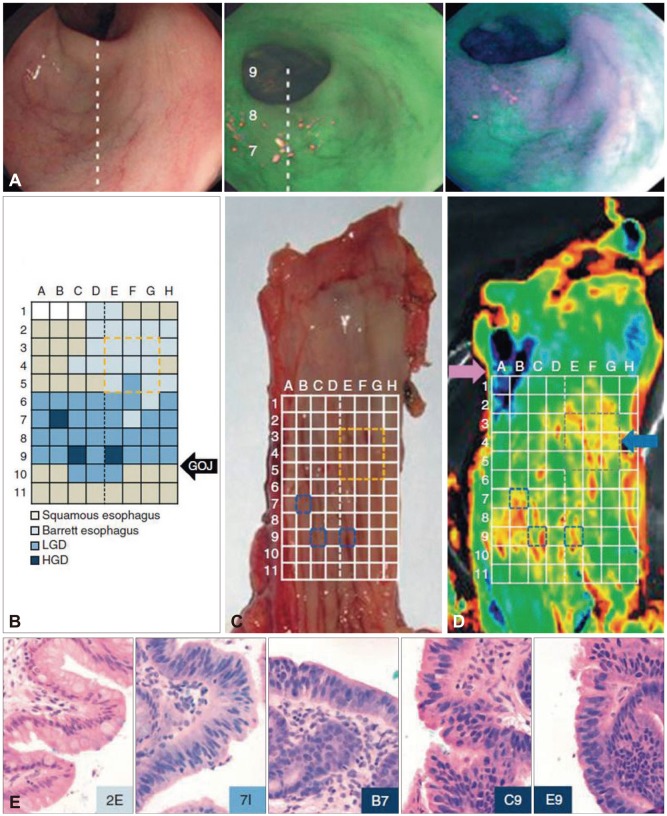
Article39. Nussbaum S, Roth HJ. Human anti-mouse antibodies: pitfalls in tumor marker measurement and strategies for enhanced assay robustness: including results with Elecsys CEA. Anticancer Res. 2000; 20(6D):5249–5252. PMID: 11326704.40. Bird-Lieberman EL, Neves AA, Lao-Sirieix P, et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nat Med. 2012; 18:315–321. PMID: 22245781.
Article41. Ozdemir V, Williams-Jones B, Glatt SJ, Tsuang MT, Lohr JB, Reist C. Shifting emphasis from pharmacogenomics to theragnostics. Nat Biotechnol. 2006; 24:942–946. PMID: 16900136.
Article42. Weissleder R. Molecular imaging: exploring the next frontier. Radiology. 1999; 212:609–614. PMID: 10478223.
Article43. Barrett T, Koyama Y, Hama Y, et al. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res. 2007; 13(22 Pt 1):6639–6648. PMID: 17982120.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic molecular imaging in inflammatory bowel disease
- Nuclear Imaging of Differentiated Thyroid Cancer: Current Status and Future Perspective
- Deep Learning in Nuclear Medicine and Molecular Imaging: Current Perspectives and Future Directions
- General Perspectives for Molecular Nuclear Imaging
- Molecular MR Imaging