J Korean Med Sci.
2015 Aug;30(8):1068-1077. 10.3346/jkms.2015.30.8.1068.
Clinical Significance of Substaging and HER2 Expression in Papillary Nonmuscle Invasive Urothelial Cancers of the Urinary Bladder
- Affiliations
-
- 1Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Colorectal Cancer Center, Kyungpook National University Medical Center, Kyungpook National University School of Medicine, Daegu, Korea.
- 4Department of Urology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 6Department of Pathology, Kyungpook National University Medical Center, Kyungpook National University School of Medicine, Daegu, Korea. gsyoon@knu.ac.kr
- KMID: 2164501
- DOI: http://doi.org/10.3346/jkms.2015.30.8.1068
Abstract
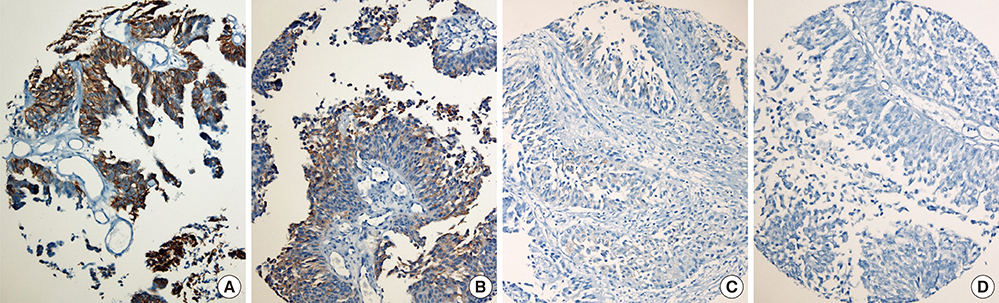
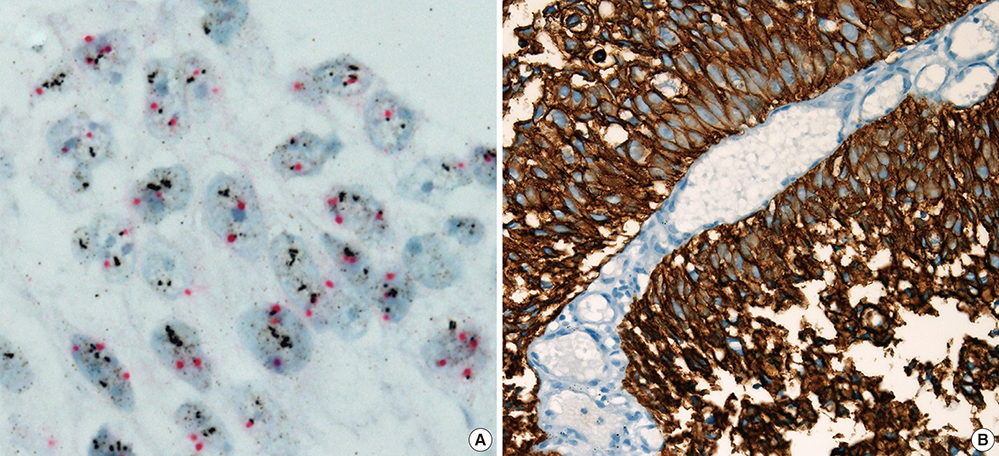
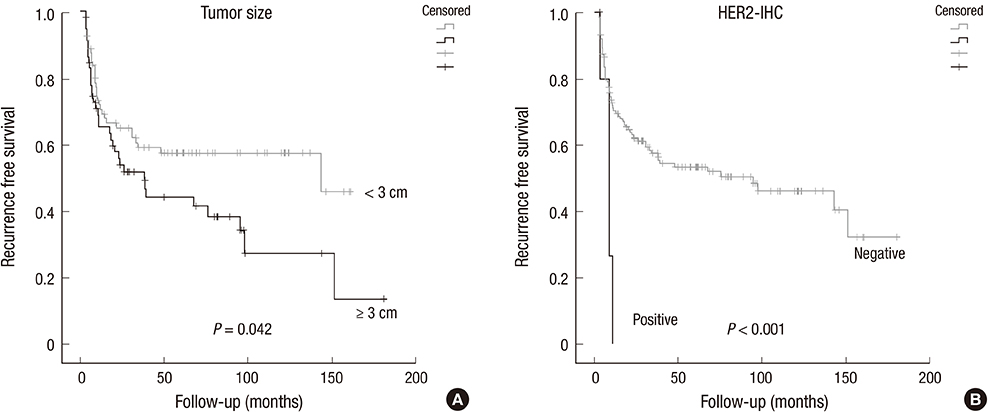
- The study aimed to verify the prognostic utility, therapeutic application and clinical benefits of tumor substaging and HER2 status in papillary non-muscle invasive bladder cancer (NMIBC). Select NMIBC transurethral resection specimens from 141 patients were used to construct tissue microarrays for assessing the substaging, HER2 protein expression by immunohistochemistry (HER2-IHC) and gene amplification by dual-color silver in situ hybridization (HER2-SISH). Substages were identified by the differing depth of tumor invasion (pTa / pT1a / pT1b / pT1c). HER2 protein expression was semiquantitatively analyzed and grouped into negative (score 0, 1+) and positive (score 2+, 3+). Other clinicopathological variables were also investigated. For NMIBC, HER2-IHC and HER2-SISH showed positive results in 6/141 (4.3%) and 4/141 (2.8%) respectively, which correlated well with tumor substaging. In multivariate analysis, substaging, HER2-IHC, and HER2-SISH were found to be independent predictors of progression-free survival (P < 0.001, P < 0.001, P = 0.031). HER2-IHC was the sole independent predictor of recurrent free survival in NMIBC (P = 0.017). It is suggested that tumor substaging and HER2 status are independent predictive markers for tumor progression or recurrence, and thus could be included in diagnostic and therapeutic management for NMIBC.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Biomarkers, Tumor/*metabolism
Carcinoma, Papillary/*metabolism/*pathology
Carcinoma, Transitional Cell/metabolism/pathology
Female
Humans
Male
Middle Aged
Neoplasm Staging
Receptor, ErbB-2/*metabolism
Reproducibility of Results
Sensitivity and Specificity
Urinary Bladder Neoplasms/*metabolism/*pathology
Young Adult
Biomarkers, Tumor
Receptor, ErbB-2
Figure
Reference
-
1. Hong SJ, Cho KS, Han M, Rhew HY, Kim CS, Ryu SB, Sul CK, Chung MK, Park TC, Kim HJ, et al. Korean Urological Oncology Society. Nomograms for prediction of disease recurrence in patients with primary Ta, T1 transitional cell carcinoma of the bladder. J Korean Med Sci. 2008; 23:428–433.2. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A, Palou Redorta J, et al. European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013; 64:639–653.3. Lee JY, Joo HJ, Cho DS, Kim SI, Ahn HS, Kim SJ. Prognostic significance of substaging according to the depth of lamina propria invasion in primary T1 transitional cell carcinoma of the bladder. Korean J Urol. 2012; 53:317–323.4. Zhao J, Xu W, Zhang Z, Song R, Zeng S, Sun Y, Xu C. Prognostic role of HER2 expression in bladder cancer: a systematic review and meta-analysis. Int Urol Nephrol. 2015; 47:87–94.5. Ha YS, Yan C, Jeong P, Kim WT, Yun SJ, Kim IY, Moon SK, Kim WJ. GSTM1 tissue genotype as a recurrence predictor in non-muscle invasive bladder cancer. J Korean Med Sci. 2011; 26:231–236.6. Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Ojea A, Portillo J, Montesinos M, Gonzalez M, Pertusa C, et al. Club Urológico Español de Tratamiento Oncológico. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: external validation of the EORTC risk tables. Eur Urol. 2011; 60:423–430.7. Lee K, Jung ES, Choi YJ, Lee KY, Lee A. Expression of pRb, p53, p16 and cyclin D1 and their clinical implications in urothelial carcinoma. J Korean Med Sci. 2010; 25:1449–1455.8. Fristrup N, Ulhøi BP, Birkenkamp-Demtröder K, Mansilla F, Sanchez-Carbayo M, Segersten U, Malmström PU, Hartmann A, Palou J, Alvarez-Múgica M, et al. Cathepsin E, maspin, Plk1, and survivin are promising prognostic protein markers for progression in non-muscle invasive bladder cancer. Am J Pathol. 2012; 180:1824–1834.9. van Rhijn BW, Zuiverloon TC, Vis AN, Radvanyi F, van Leenders GJ, Ooms BC, Kirkels WJ, Lockwood GA, Boevé ER, Jöbsis AC, et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur Urol. 2010; 58:433–441.10. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. American Society of Clinical Oncology. College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014; 138:241–256.11. Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, Bangs CD, Cherry AM, Pai RK. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol. 2012; 20:13–24.12. Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010; 21:815–819.13. Bolenz C, Shariat SF, Karakiewicz PI, Ashfaq R, Ho R, Sagalowsky AI, Lotan Y. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int. 2010; 106:1216–1222.14. Chen PC, Yu HJ, Chang YH, Pan CC. Her2 amplification distinguishes a subset of non-muscle-invasive bladder cancers with a high risk of progression. J Clin Pathol. 2013; 66:113–119.15. Olsson H, Fyhr IM, Hultman P, Jahnson S. HER2 status in primary stage T1 urothelial cell carcinoma of the urinary bladder. Scand J Urol Nephrol. 2012; 46:102–107.16. Brimo F, Wu C, Zeizafoun N, Tanguay S, Aprikian A, Mansure JJ, Kassouf W. Prognostic factors in T1 bladder urothelial carcinoma: the value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion. Hum Pathol. 2013; 44:95–102.17. Hu Z, Mudaliar K, Quek ML, Paner GP, Barkan GA. Measuring the dimension of invasive component in pT1 urothelial carcinoma in transurethral resection specimens can predict time to recurrence. Ann Diagn Pathol. 2014; 18:49–52.18. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer;2010. p. 497–502.19. Lopez-Beltran A, Sauter G, Gasser T, Hartmann A, Schmitz-Drager BJ, Helpap B, Ayala AG, Tamboli P, Knowles MA, Sidransky D, et al. Infiltrating urothelial carcinoma. In : Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC Press;2004. p. 93–109. (World Health Organization Classification of Tumors; vol 6).20. van Rhijn BW, van der Kwast TH, Alkhateeb SS, Fleshner NE, van Leenders GJ, Bostrom PJ, van der Aa MN, Kakiashvili DM, Bangma CH, Jewett MA, et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol. 2012; 61:378–384.21. Bellmunt J, Werner L, Bamias A, Fay AP, Park RS, Riester M, Selvarajah S, Barletta JA, Berman DM, de Muga S, et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med. 2015; 4:844–852.22. Lim SJ, Cantillep A, Carpenter PM. Validation and workflow optimization of human epidermal growth factor receptor 2 testing using INFORM HER2 dual-color in situ hybridization. Hum Pathol. 2013; 44:2590–2596.23. Caner V, Turk NS, Duzcan F, Tufan NL, Kelten EC, Zencir S, Dodurga Y, Bagci H, Duzcan SE. No strong association between HER-2/neu protein overexpression and gene amplification in high-grade invasive urothelial carcinomas. Pathol Oncol Res. 2008; 14:261–266.24. Fleischmann A, Rotzer D, Seiler R, Studer UE, Thalmann GN. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011; 60:350–357.25. Schneider SA, Sukov WR, Frank I, Boorjian SA, Costello BA, Tarrell RF, Thapa P, Houston Thompson R, Tollefson MK, Jeffrey Karnes R, et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod Pathol. 2014; 27:758–764.26. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014; 507:315–322.27. Burandt E, Schreiber M, Stein A, Minner S, Clauditz TS, Bokemeyer C, Jänicke F, Fisch M, Izbicki JR, Knecht R, et al. Continuous tissue microarray based identification of cancers with homogeneous target expression for successful targeted therapy in clinical routine practice. Genes Chromosomes Cancer. 2014; 53:228–239.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autophagy and urothelial carcinoma of the bladder: A review
- Expression of Survivin, HSP90, Bcl-2 and Bax Proteins in N-butyl-N-(4-hydroxybutyl)nitrosamine-induced Rat Bladder Carcinogenesis
- Papillary Urothelial Tumor of Bladder in 5-year-old Girl
- High-Grade Urothelial Carcinoma of the Bladder in a Child
- Immunophenotypic and molecular changes during progression of papillary urothelial carcinoma