J Korean Med Sci.
2015 Apr;30(4):426-434. 10.3346/jkms.2015.30.4.426.
Safety and Efficacy of a Novel, Fenestrated Aortic Arch Stent Graft with a Preloaded Catheter for Supraaortic Arch Vessels: An Experimental Study in Swine
- Affiliations
-
- 1Department of Thoracic Surgery, College of Medicine, Pusan National University, Medical Research Institute, Pusan National University Hospital, Busan, Korea.
- 2Division of Cardiology, Department of Internal Medicine, College of Medicine, Pusan National University, Medical Research Institute, Pusan National University Hospital, Busan, Korea. glaraone@hanmail.net
- KMID: 2164450
- DOI: http://doi.org/10.3346/jkms.2015.30.4.426
Abstract
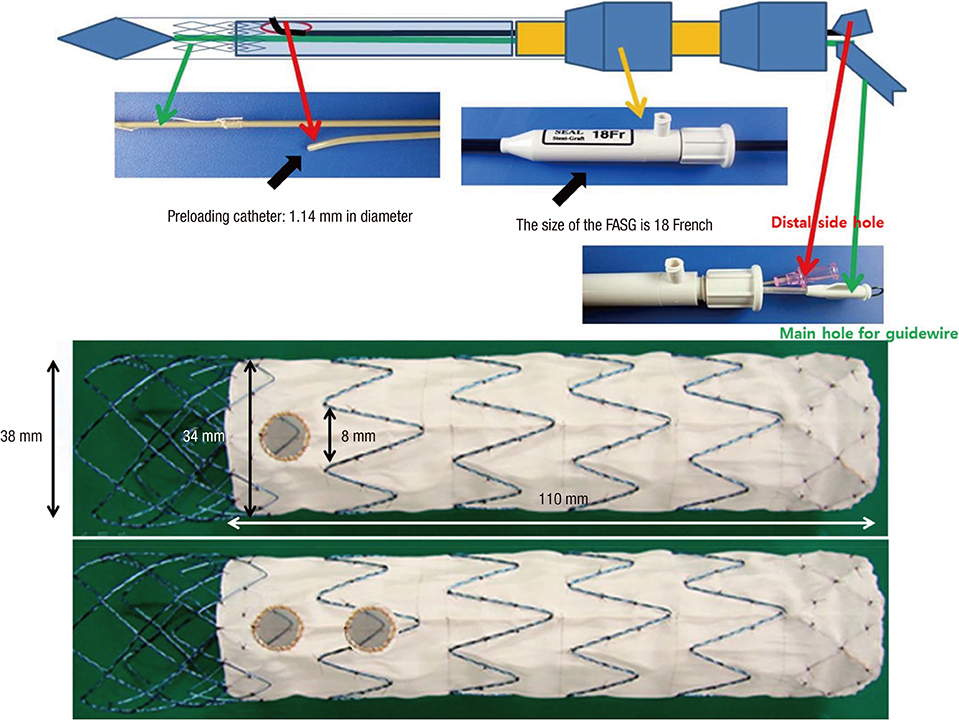
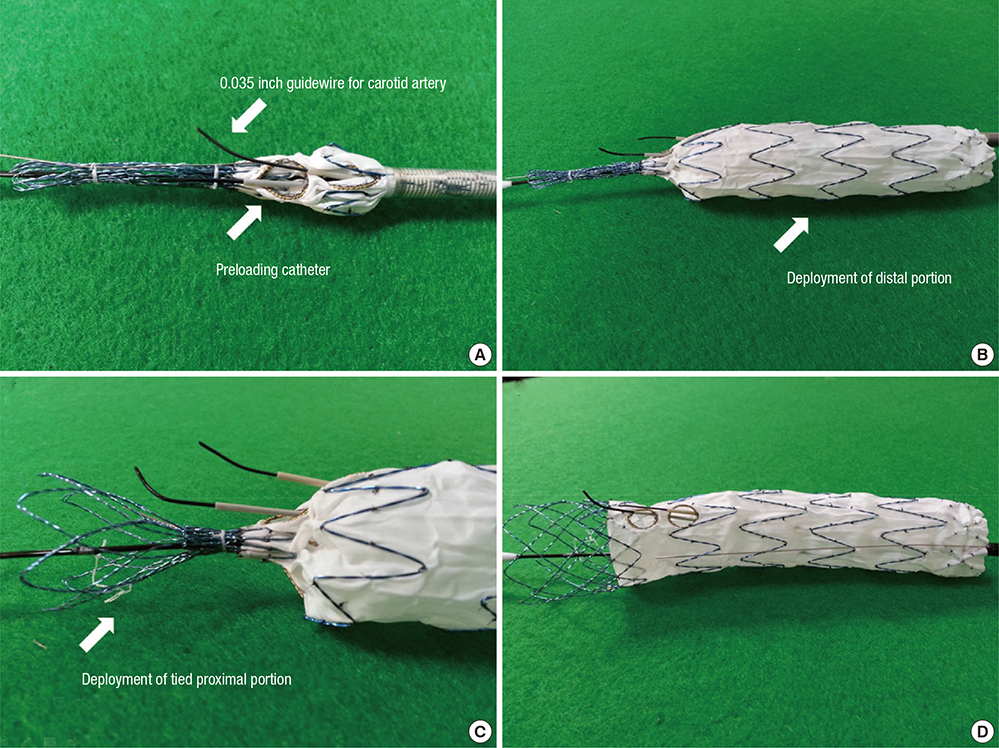
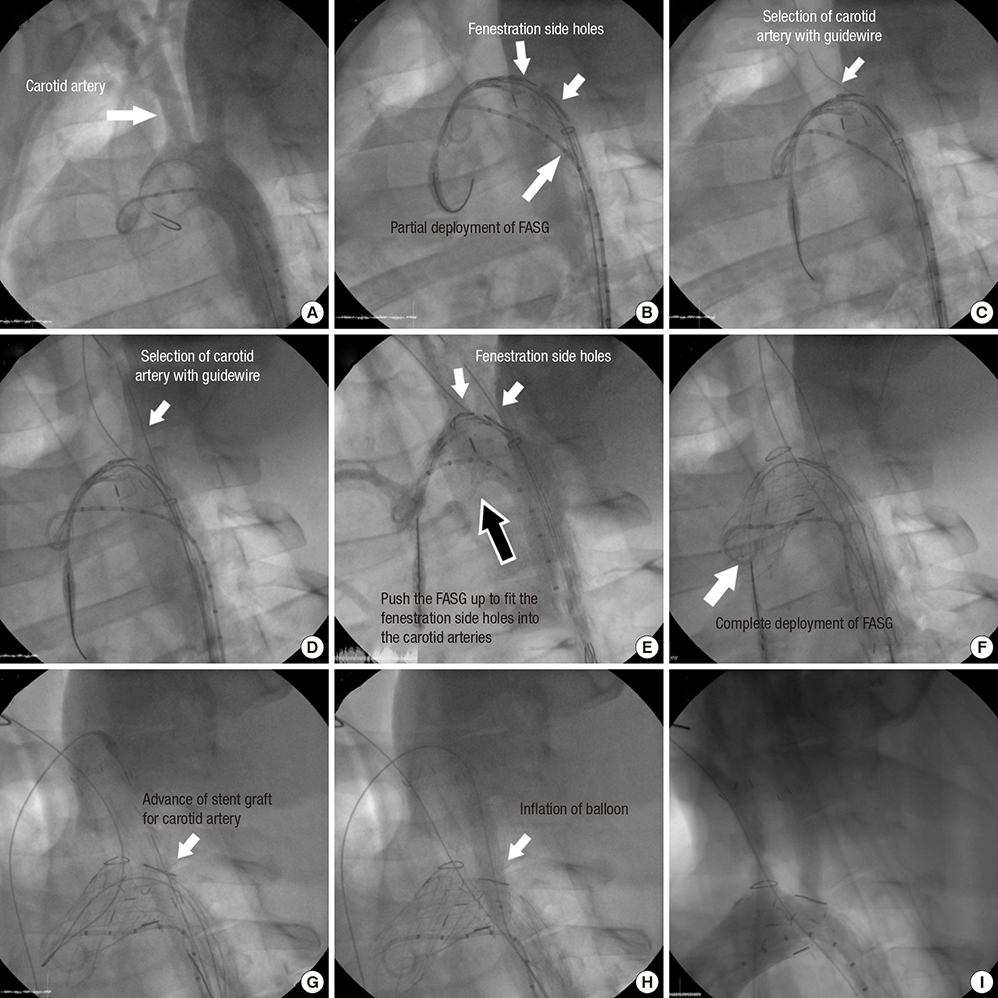
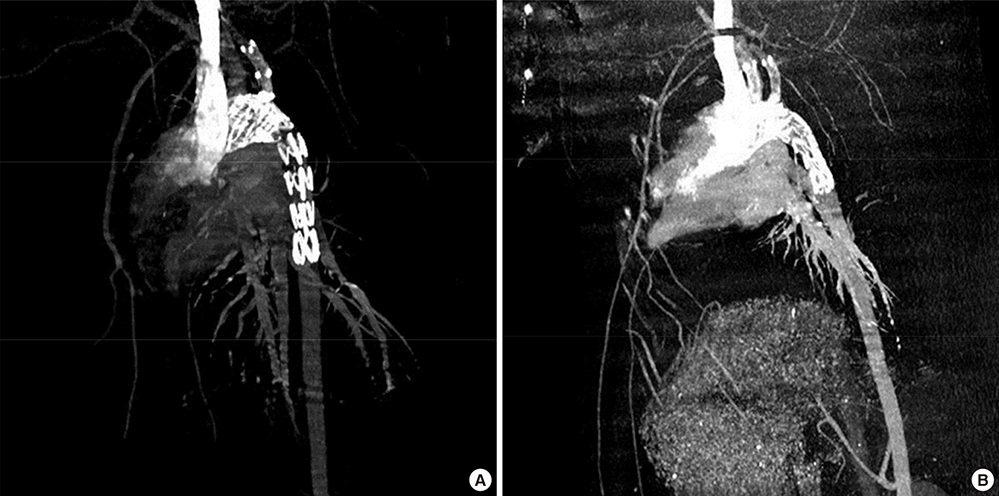
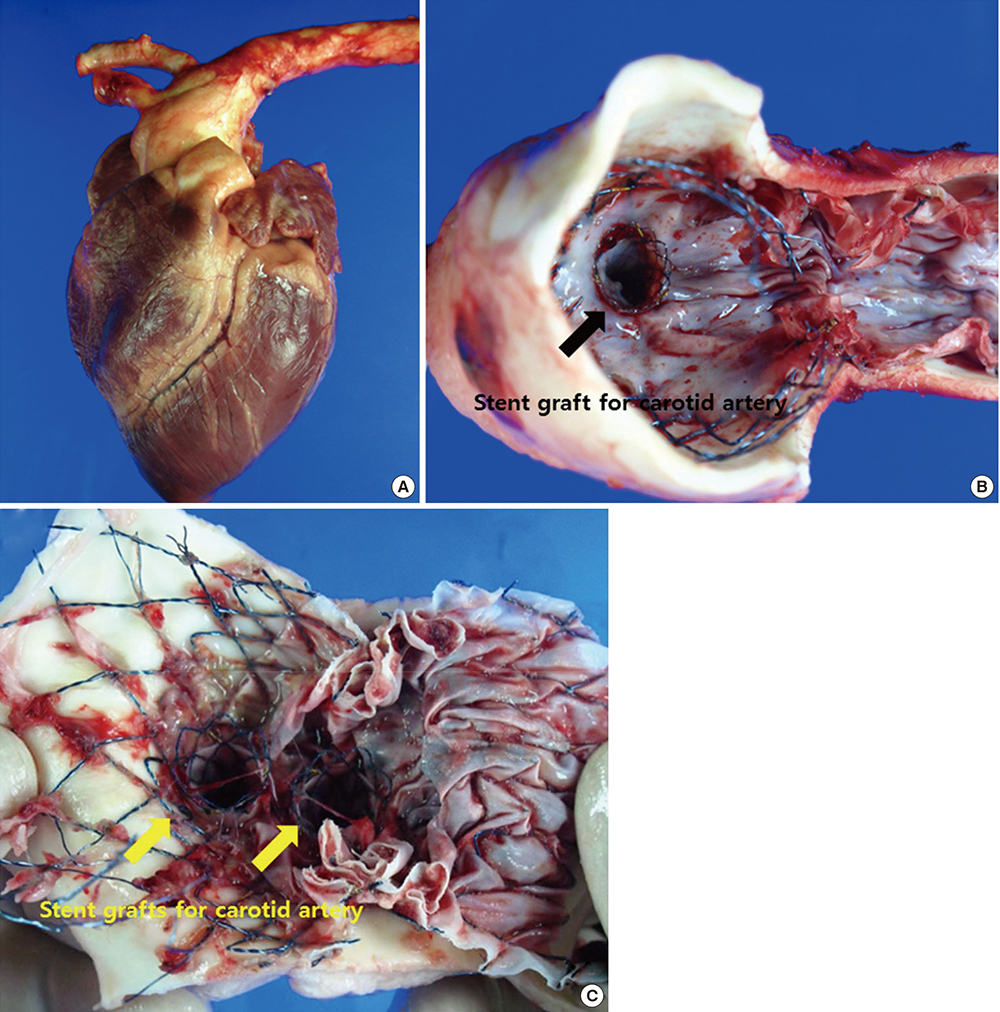
- Thoracic endovascular aortic repair (TEVAR) shows limitations in cases in which the aortic pathology involves the aortic arch. The study aims were to test a fenestrated aortic arch stent graft (FASG) with a preloaded catheter for the supraaortic arch vessels and to perform a preclinical study in swine to evaluate the safety and efficacy of this device. Six FASGs with 1 preloaded catheter and 5 FASGs with 2 preloaded catheters were advanced through the iliac artery in 11 swines. The presence of endoleaks and the patency and deformity of the grafts were examined with computed tomography (CT) at 4 weeks postoperatively. A postmortem examination was performed at 8 weeks. The mean procedure time for the one and two FASG groups was 30.2 (27.9-34.5) min and 43.1 (39.2-53.7) min. The mean time for the selection of the carotid artery was 4.8 (4.2-5.5) min and 6.2 (4.6-9.4) min. Major adverse event was observed in one of 11 pigs. One pig died at 4 weeks likely because of the effects of the high dose of ketamine, while the remaining 10 pigs survived 8-week. For both the one and two FASG groups, no endoleaks, no disconnection, no occlusion of the stent grafts were observed in the CT findings and the postmortem gross findings. The procedure with the FASG could be performed safely in a relatively short procedure time and involved an easy technique. The FASG is found to be safe and convenient in this preclinical study with swine.
MeSH Terms
Figure
Reference
-
1. Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, Hirano T, Takeda K, Yada I, Miller DC. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999; 340:1546–1552.2. Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994; 331:1729–1734.3. Greenberg R, Resch T, Nyman U, Lindh M, Brunkwall J, Brunkwall P, Malina M, Koul B, Lindblad B, Ivancev K. Endovascular repair of descending thoracic aortic aneurysms: an early experience with intermediate-term follow-up. J Vasc Surg. 2000; 31:147–156.4. Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T, et al. ; INSTEAD Trial. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009; 120:2519–2528.5. Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012; 33:26–35b.6. Shah AA, Barfield ME, Andersen ND, Williams JB, Shah JA, Hanna JM, McCann RL, Hughes GC. Results of thoracic endovascular aortic repair 6 years after United States Food and Drug Administration approval. Ann Thorac Surg. 2012; 94:1394–1399.7. Zhang H, Wang ZW, Zhou Z, Hu XP, Wu HB, Guo Y. Endovascular stent-graft placement or open surgery for the treatment of acute type B aortic dissection: a meta-analysis. Ann Vasc Surg. 2012; 26:454–461.8. Narayan P, Wong A, Davies I, Angelini GD, Bryan AJ, Wilde P, Murphy GJ. Thoracic endovascular repair versus open surgical repair - which is the more cost-effective intervention for descending thoracic aortic pathologies. Eur J Cardiothorac Surg. 2011; 40:869–874.9. Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP. Gore TAG Investigators. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008; 47:912–918.10. Lee SH, Chung CH, Jung SH, Lee JW, Shin JH, Ko Ky, Yoon HK, Choo SJ. Midterm outcomes of open surgical repair compared with thoracic endovascular repair for isolated descending thoracic aortic disease. Korean J Radiol. 2012; 13:476–482.11. Al-Nouri O, Moeller C, Borrowdale R, Milner R. Late complication of thoracic endovascular stent-grafting. Ann Vasc Surg. 2011; 25:982.e1–982.e4.12. Lee CJ, Rodriguez HE, Kibbe MR, Malaisrie SC, Eskandari MK. Secondary interventions after elective thoracic endovascular aortic repair for degenerative aneurysms. J Vasc Surg. 2013; 57:1269–1274.13. Ullery BW, McGarvey M, Cheung AT, Fairman RM, Jackson BM, Woo EY, Desai ND, Wang GJ. Vascular distribution of stroke and its relationship to perioperative mortality and neurologic outcome after thoracic endovascular aortic repair. J Vasc Surg. 2012; 56:1510–1517.14. Grabenwöger M, Alfonso F, Bachet J, Bonser R, Czerny M, Eggebrecht H, Evangelista A, Fattori R, Jakob H, Lönn L, et al. European Association for Cardio-Thoracic Surgery (EACTS). European Society of Cardiology (ESC). European Association of Percutaneous Cardiovascular Interventions (EAPCI). Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2012; 42:17–24.15. Lee KN, Lee HC, Park JS, Kim BW, Cha KS, Kim SP, Lee CW, Kim HK. The modified chimney technique with a thoracic aortic stent graft to preserve the blood flow of the left common carotid artery for treating descending thoracic aortic aneurysm and dissection. Korean Circ J. 2012; 42:360–365.16. Sun Z, Mwipatayi BP, Allen YB, Hartley DE, Lawrence-Brown MM. Multislice CT angiography of fenestrated endovascular stent grafting for treating abdominal aortic aneurysms: a pictorial review of the 2D/3D visualizations. Korean J Radiol. 2009; 10:285–293.17. Inoue K, Hosokawa H, Iwase T, Sato M, Yoshida Y, Ueno K, Tsubokawa A, Tanaka T, Tamaki S, Suzuki T. Aortic arch reconstruction by transluminally placed endovascular branched stent graft. Circulation. 1999; 100:Ii316–Ii321.18. Schneider DB, Curry TK, Reilly LM, Kang JW, Messina LM, Chuter TA. Branched endovascular repair of aortic arch aneurysm with a modular stent-graft system. J Vasc Surg. 2003; 38:855.19. Ahanchi SS, Almaroof B, Stout CL, Panneton JM. In situ laser fenestration for revascularization of the left subclavian artery during emergent thoracic endovascular aortic repair. J Endovasc Ther. 2012; 19:226–230.20. Wei G, Xin J, Yang D, Liu X, Tai Y, Zhang H, Liang F, Zhang G. A new modular stent graft to reconstruct aortic arch. Eur J Vasc Endovasc Surg. 2009; 37:560–565.21. Yokoi Y, Azuma T, Yamazaki K. Advantage of a precurved fenestrated endograft for aortic arch disease: simplified arch aneurysm treatment in Japan 2010 and 2011. J Thorac Cardiovasc Surg. 2013; 145:S103–S109.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and Efficacy of an Aortic Arch Stent Graft with Window-Shaped Fenestration for Supra-Aortic Arch Vessels: an Experimental Study in Swine
- Hybrid Procedure for Aortic Arch Repair: Arch Vessels Debranching with Supraaortic Revascularization Followed by Endovascular Aortic Stent Grafting
- Endovascular Repair of Thoracic Aortic Aneurysm Using a Custom-made Fenestrated Stent Graft to Preserve the Left Subclavian Artery
- Hybrid Method for Stent-graft Insertion in a Patient with a Thoracic Aortic Aneurysm Involving the Aortic Arch: A case report
- Aortic Arch Debranching and Antegrade Stent Graft Placement in an Expanding Distal Dissecting Aneurysm after Repair of an Acute Type I Aortic Dissection