J Korean Med Sci.
2015 Apr;30(4):353-359. 10.3346/jkms.2015.30.4.353.
Vaccination Policy in Korean Armed Forces: Current Status and Future Challenge
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea.
- 2Department of Internal Medicine, The Armed Forces Capital Hospital, Seongnam, Korea. choekw@snu.ac.kr
- 3Department of Preventive Medicine, The Armed Forces Medical Command, Seongnam, Korea.
- 4Division of Infectious Diseases, Department of Internal Medicine, Korea University Kuro Hospital, Seoul, Korea.
- KMID: 2164440
- DOI: http://doi.org/10.3346/jkms.2015.30.4.353
Abstract
- Infectious diseases have historically resulted in suspended or cancelled military operations. Vaccination for disease prevention is a critical component of the military's force readiness doctrine. Until recently, Korea had not recognized the importance of vaccinating military personnel. However, a 2011 meningococcal disease outbreak at an army recruit training center led to dramatic changes in the paradigm of traditional medical practice in the Korean armed forces. A new vaccination policy was formed by a 2012 Military Healthcare Service Act. Since then, Neisseria meningitidis, hepatitis A, and measles-mumps-rubella vaccines have been routinely administered to all new recruits early in basic training to ensure protection against these diseases. All active-duty soldiers also receive seasonal influenza vaccination annually. Despite quantitative improvements in vaccination policies, several instances of major infectious diseases and adverse vaccine reactions have threatened soldier health. In the future, vaccination policies in the Korean armed forces should be based on epidemiologic data and military medical research for vaccine use and safety management.
Keyword
MeSH Terms
Figure
Cited by 3 articles
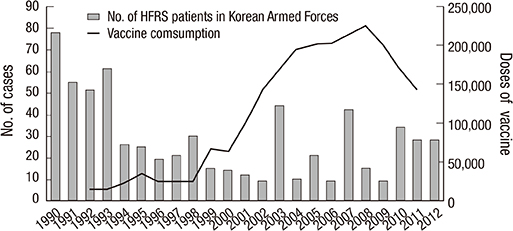
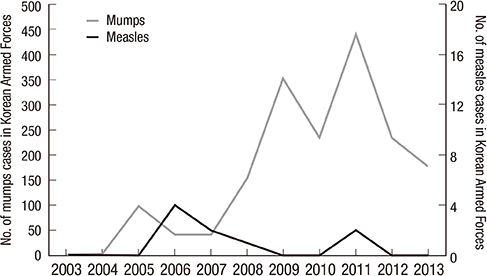
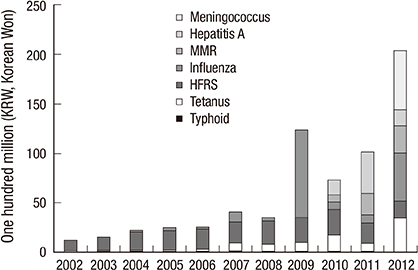
-
국내 일개 대학 병원 근무 의료인의 A형간염항체 양성률에 대한 연구
Seul Ki Ji, So Hee Jang, Min Hee Park, Ji Eun Lee, Hye Sook Jeong, Joonhong Park, Seung Beom Han, Yunmi Yi, Sun Hee Park
Korean J Healthc Assoc Infect Control Prev. 2020;25(1):54-59. doi: 10.14192/kjicp.2020.25.1.54.Comparison of Immune Responses to Two Quadrivalent Meningococcal Conjugate Vaccines (CRM197 and Diphtheria Toxoid) in Healthy Adults
Han Wool Kim, Soyoung Lee, Ji Hyen Lee, So-Youn Woo, Kyung-Hyo Kim
J Korean Med Sci. 2019;34(23):. doi: 10.3346/jkms.2019.34.e169.Investigation of a Mumps Outbreak in a Dental Clinic at a University Hospital
Jeong Eun Lee, Soon Ok Lee, Jin Suk Kang, Jongyoun Yi, Kye-Hyung Kim
Infect Chemother. 2019;51(3):256-262. doi: 10.3947/ic.2019.51.3.256.
Reference
-
1. Siler JF. The U.S. Army Medical Department in the World War. Vol 9: Communicable and other diseases. Washinnton: U.S. Government Printing Office;1928. p. 66–70.2. Gray GC, Feighner B, Trump DH, Berg SW, Zajdowicz MJ, Zajdowicz TR. Disease spread by close personal contact. In : Lenhart MK, Lounsbury DE, editors. Military Preventive Medicine: Mobilization and Deployment, Vol 2. Washington DC: Borden Institute;2005. p. 1127–1128.3. Chun CH, Kim SW, Kim WD, Yoon HJ. Changing patterns of epidemiology and death: Causes on epidemic hemorrhagic fever in Korea. Korean J Infect Dis. 1976; 8:58–74.4. Johnson KM. Hantaviruses: history and overview. Curr Top Microbiol Immunol. 2001; 256:1–14.5. Murray CK, Horvath LL. An approach to prevention of infectious diseases during military deployments. Clin Infect Dis. 2007; 44:424–430.6. Artenstein AW, Opal JM, Opal SM, Tramont EC, Peter G, Russell PK. History of U.S. military contributions to the study of vaccines against infectious diseases. Mil Med. 2005; 170:3–11.7. Mancuso JD, Tobler SK, Eick AA, Keep LW. Active tuberculosis and recent overseas deployment in the U.S. military. Am J Prev Med. 2010; 39:157–163.8. Finnie TJ, Copley VR, Hall IM, Leach S. An analysis of influenza outbreaks in institutions and enclosed societies. Epidemiol Infect. 2014; 142:107–113.9. Duron S, Mayet A, Lienhard F, Haus-Cheymol R, Verret C, Védy S, Le Guen P, Berbineau L, Brisou P, Dubrous P, et al. MISS staff. The French Military influenza surveillance system (MISS): overview of epidemiological and virological results during four influenza seasons--2008-2012. Swiss Med Wkly. 2013; 143:w13848.10. Heo JY, Yoon CG, Kim KH, Choe KW. Incidence of influenza-like illness in Korean military personnel: comparison between military and community population. In : International Interscience Conference on Infection and Chemotherapy; 2013; Seoul, Korea.11. Lee SO, Ryu SH, Park SJ, Ryu J, Woo JH, Kim YS. Meningococcal disease in the republic of Korea army: incidence and serogroups determined by PCR. J Korean Med Sci. 2003; 18:163–166.12. Kang CI, Choi CM, Kim DH, Kim CH, Lee DJ, Kim HB, Kim NJ, Oh MD, Choe KW. Pulmonary tuberculosis in young Korean soldiers: incidence, drug resistance and treatment outcomes. Int J Tuberc Lung Dis. 2006; 10:970–974.13. Lee SW, Jang YS, Park CM, Kang HY, Koh WJ, Yim JJ, Jeon K. The role of chest CT scanning in TB outbreak investigation. Chest. 2010; 137:1057–1064.14. Broderick MP, Faix DJ, Hansen CJ, Blair PJ. Trends in meningococcal disease in the United States military, 1971-2010. Emerg Infect Dis. 2012; 18:1430–1437.15. Kang CI, Choi CM, Park TS, Lee DJ, Oh MD, Choe KW. Incidence of herpes zoster and seroprevalence of varicella-zoster virus in young adults of South Korea. Int J Infect Dis. 2008; 12:245–247.16. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999; 5:379–385.17. Song JY, Chun BC, Kim SD, Baek LJ, Kim SH, Sohn JW, Cheong HJ, Kim WJ, Park SC, Kim MJ. Epidemiology of hemorrhagic fever with renal syndrome in endemic area of the Republic of Korea, 1995-1998. J Korean Med Sci. 2006; 21:614–620.18. Kim SS, Kim JS. Epiderniologic characteristics and trends of leptospirosis in Korea by literature review. Korean J Epidemiol. 1994; 16:66–83.19. Sames WJ, Kim HC, Klein TA. Perspectives on scrub typhus, tick-borne pathogens, and hantavirus in the Republic of Korea. US Army Med Dep J. 2009; 40–49.20. Baker MS, Ryals PA. The medical department in military operations other than war. Part I. Planning for deployment. Mil Med. 1999; 164:572–579.21. Ryals PA, Baker MS. Military medicine in operations other than war. Part II: Humanitarian relief missions for Naval Reserve fleet hospitals. Mil Med. 1996; 161:502–504.22. Ministry of National Defense. Current overseas deployment of Korean armed forces in five Asian and African counties 2013. accessed on 1 September 2014. Available at http://www.index.go.kr/egams/stts/jsp/potal/stts/PO_STTS_IdxMain.jsp?idx_ cd=1715&bbs=INDX_001&clas_div=C&rootKey=1.48.0.23. Korean Center for Diseases Control. Current preparedness against bioterrorism using smallpox in Korea. accessed on 1 September 2014. Available at http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0037-MNU1380&cid=12467.24. Rusnak JM, Boudreau EF, Hepburn MJ, Martin JW, Bavari S. Medical Counter measures. In : Lenhart MK, Lounsbury DE, editors. Medical Aspects of Biological Warfare. Washington DC: Borden Institute;2007. p. 465–467.25. Korean Center for Diseases Control. Trends in development of Anthrax Vaccine. accessed on 1 September 2014. Available at http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0037-MNU1380&cid=12372.26. Artenstein AW. Vaccines for military use. Vaccine. 2009; 27:D16–D22.27. Lee SO. Commencement of the meningococcal vaccination for the republic of Korea army. Infect Chemother. 2013; 45:113–115.28. Ministry of Goverment Legislation. Act on Military Healthcare Service. accessed on 1 September 2014. Available at http://www.law.go.kr/lsInfoP.do?lsiSeq=124038&efYd=20120922#0000.29. Ministry of National Defense. Manual for military immunization. 2010.30. Korea Centers for Disease Control and Prevention. Disease web statistics system. accessed on 1 September 2014. Available at http://is.cdc.go.kr/nstat/index.jsp.31. Armed Forces Medical Research Institute. Infect Dis Wkly Rep. 2013; 7:3–4.32. Lee SY, Kim JS, Ahn JH, Choi JH, Ma SH, Park JS, Kim HM, Kang JH. Immunoassay of Diphtheria and Tetanus according to Ages. Infect Chemother. 2012; 44:62–66.33. Kim CK, Shin JH. Qualitative analysis of the tetanus antibody in Korean Army Personnel after visiting a Tertiary Armed Forces Hospital. J Korean Soc Traumatol. 2007; 20:65–71.34. Chu YK, Gligic A, Tomanovic S, Bozovjc B, Obradovic M, Woo YD, An CN, Kim H, Jiang YS, Park SC, et al. A field efficacy trial of inactivated Hantaan Virus Vaccine (Hantavax(TM)) against hemorrhagic fever with renal syndrome (HFRS) in the endemic areas of Yugoslavia from 1996 to 1998. J Korean Soc Virol. 1999; 29:55–64.35. Sohn YM, Rho HO, Park MS, Kim JS, Summers PL. Primary humoral immune responses to formalin inactivated hemorrhagic fever with renal syndrome vaccine (Hantavax): consideration of active immunization in South Korea. Yonsei Med J. 2001; 42:278–284.36. Park K, Kim CS, Moon KT. Protective effectiveness of hantavirus vaccine. Emerg Infect Dis. 2004; 10:2218–2220.37. Rosenstein NE, Perkins BA, Stephens DS, Lefkowitz L, Cartter ML, Danila R, Cieslak P, Shutt KA, Popovic T, Schuchat A, et al. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J Infect Dis. 1999; 180:1894–1901.38. Gunzenhauser JD. Communicable Disease Control in Basic Training: Programmatic Aspect. In : Kelley PW, editor. Military Preventive Medicine: Mobilization and Deployment, Vol 1. Washington DC: Borden Institute;2003. p. 145–150.39. Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006; 28:3–26.40. Meningococcal disease and college students. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000; 49:13–20.41. Bilukha OO, Rosenstein N. National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005; 54:1–21.42. Shepard CW, Ortega-Sanchez IR, Scott RD 2nd, Rosenstein NE. ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics. 2005; 115:1220–1232.43. Park HS, Chun YI. Vaccination effect on pharyngeal carrier rate of Neis seria meningitidis and its serogroups in Korean Army Recruits. J Korean Mil Med Assoc. 1992; 23:105–115.44. Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001; 344:1378–1388.45. Sim SH, Heo JY, Kim EC, Choe KW. A case of meningococcal sepsis and meningitis with complement 7 deficiency in a military trainee. Infect Chemother. 2013; 45:94–98.46. Pazzaglia G, Pasternack M. Recent trends of pneumonia morbidity in US Naval personnel. Mil Med. 1983; 148:647–651.47. Kim HY. Seven Mortality cases by pneumonia in military recruits during 2004-05 influenza season. Nocutnews. 2005. 09. 22. accessed on 1 September 2014. Available at http://www.nocutnews.co.kr/show.asp?idx=74568.48. Seng W, Maeng S. Acute Hepatitis A in some brigade. J Korean Mil Med Assoc. 2008; 39:130–135.49. Han SH, Lee SH, Roh BJ, Shim SC, Cho SC, Sohn JH, Lee DH, Kee CS. An outbreak of hepatitis A in South Korean military personnel: a clinical and epidemiologic study. Korean J Hepatol. 2001; 7:392–400.50. Kang CI, Choi CM, Park TS, Lee DJ, Oh MD, Choe KW. Incidence and seroprevalence of hepatitis A virus infections among young Korean soldiers. J Korean Med Sci. 2007; 22:546–548.51. Armed Forces Medical Command. Defense Medical Defense Medical Statics Information System(DMSIS). 2013.52. Heo JY, Song JY, Noh JY, Seo YB, Kim IS, Choi WS, Kim WJ, Cho GJ, Hwang TG, Cheong HJ. Low level of immunity against hepatitis A among Korean adolescents: vaccination rate and related factors. Am J Infect Control. 2013; 41:e97–e100.53. Orr N, Klement E, Gillis D, Sela T, Kayouf R, Derazne E, Grotto I, Balicer R, Huerta M, Aviram L, et al. Long-term immunity in young adults after a single dose of inactivated Hepatitis A vaccines. Vaccine. 2006; 24:4328–4332.54. Weese C, Clark K, Goldman D, Underwood PK. Diseases Controlled Primarily by Vaccination. In : Kelley PW, editor. Military Preventive Medicine: Mobilization and Deployment. Washington, D. C.: Borden Institute, Walter Reed Army Medical Center;2005. p. 1215–1223.55. Choi YH, Kim BN. Measle-Mumps-Rubella. Korean Society of Infectious Diseases. Vaccination for adult. 2nd ed. Seoul: M.I.P;2012. p. 236–253.56. Park Y, Lee H, Lee Y, Hwang J, Kim K, Kim J. Seroprevalence of Mumps and Seroconversion Rate after MMR Vaccination in ROK Army. Koean J Mil Med Assoc. 2012; 43:29–34.57. Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature. 1985; 318:323–329.58. Gobet A, Mayet A, Journaux L, Dia A, Aigle L, Dubrous P, Michel R. Mumps among highly vaccinated people: investigation of an outbreak in a French Military Parachuting Unit, 2013. J Infect. 2014; 68:101–102.59. Park DW, Nam MH, Kim JY, Kim HJ, Sohn JW, Cho Y, Song KJ, Kim MJ. Mumps outbreak in a highly vaccinated school population: assessment of secondary vaccine failure using IgG avidity measurements. Vaccine. 2007; 25:4665–4670.60. Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, Hunt K, Finley CG, Leschinsky DP, O'Keefe AL, et al. Recent resurgence of mumps in the United States. N Engl J Med. 2008; 358:1580–1589.61. Dayan GH, Rubin S. Mumps outbreaks in vaccinated populations: are available mumps vaccines effective enough to prevent outbreaks? Clin Infect Dis. 2008; 47:1458–1467.62. John HD. Acute Respiratory Disease Among New Recruits. Am J Public Health Nations Health. 1946; 36:439–450.63. Heo JY, Lee JE, Kim HK, Choe KW. Acute lower respiratory tract infections in soldiers, South Korea, April 2011-March 2012. Emerg Infect Dis. 2014; 20:875–877.64. Hoke CH Jr, Snyder CE Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine. 2013; 31:1623–1632.65. Potter RN, Cantrell JA, Mallak CT, Gaydos JC. Adenovirus-associated deaths in US military during postvaccination period, 1999-2010. Emerg Infect Dis. 2012; 18:507–509.66. Gray GC, Goswami PR, Malasig MD, Hawksworth AW, Trump DH, Ryan MA, Schnurr DP. Adult adenovirus infections: loss of orphaned vaccines precipitates military respiratory disease epidemics. For the Adenovirus Surveillance Group. Clin Infect Dis. 2000; 31:663–670.67. Malarkey MA, Baylor NW. March 16, 2011 Approval Letter - Adenovirus Type 4 and Type 7 Vaccine, Live, Oral. Silver Spring: US Food and Drug Administration;accessed on 1 September 2014. Available at http://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm247511.htm.68. Heo JY, Kim HK, Cha YJ, Lee JE, Shim YS, Choe KW. A clinical features of severe adenovirus pneumonia among members of the Korea Military: a case series. Infect Chemother. 2012; 44:372–376.69. Choe YJ, Park YJ, Jung C, Bae GR, Lee DH. National pertussis surveillance in South Korea 1955-2011: epidemiological and clinical trends. Int J Infect Dis. 2012; 16:e850–e854.70. Armed Forces Health Surveillance Center (AFHSC). Pertussis diagnoses among service members and other beneficiaries of the U.S. Military Health System, January 2005-June 2012. MSMR. 2012; 19:14–17.71. Grabenstein JD, Nevin RL. Mass immunization programs: principles and standards. Curr Top Microbiol Immunol. 2006; 304:31–51.72. Chung EH. Vaccine allergies. Clin Exp Vaccine Res. 2014; 3:50–57.73. Korea Centers for Disease Control and Prevention. Evaluation of national immunization registry, 2002-2010. accessed on 1 September 2014. Available at http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0037-MNU1380&cid=12634.74. Linkins RW. Immunization registries: progress and challenges in reaching the 2010 national objective. J Public Health Manag Pract. 2001; 7:67–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Parsonage-Turner Syndrome Following Typhoid Vaccination
- Analyses of Confirmed COVID-19 Cases Among Korean Military Personnel After Mass Vaccination
- Qualitative Analysis of the Tetanus Antibody in Korean Army personnel after Visiting a Tertiary Armed Forces Hospital
- Vaccination Rate and Seroepidemiology of Hepatitis A in Chronic-Hepatitis-B-Infected Individuals in the Korean Army
- Varicella vaccination: Worldwide status and p rovisional updated recommendation in Korea