Int J Stem Cells.
2016 May;9(1):21-30. 10.15283/ijsc.2016.9.1.21.
Recent Stem Cell Advances: Cord Blood and Induced Pluripotent Stem Cell for Cardiac Regeneration- a Review
- Affiliations
-
- 1Department of Pharmacology, Satara College of Pharmacy, Degaon, Satara (MH), India. smedhekar5@gmail.com
- KMID: 2164157
- DOI: http://doi.org/10.15283/ijsc.2016.9.1.21
Abstract
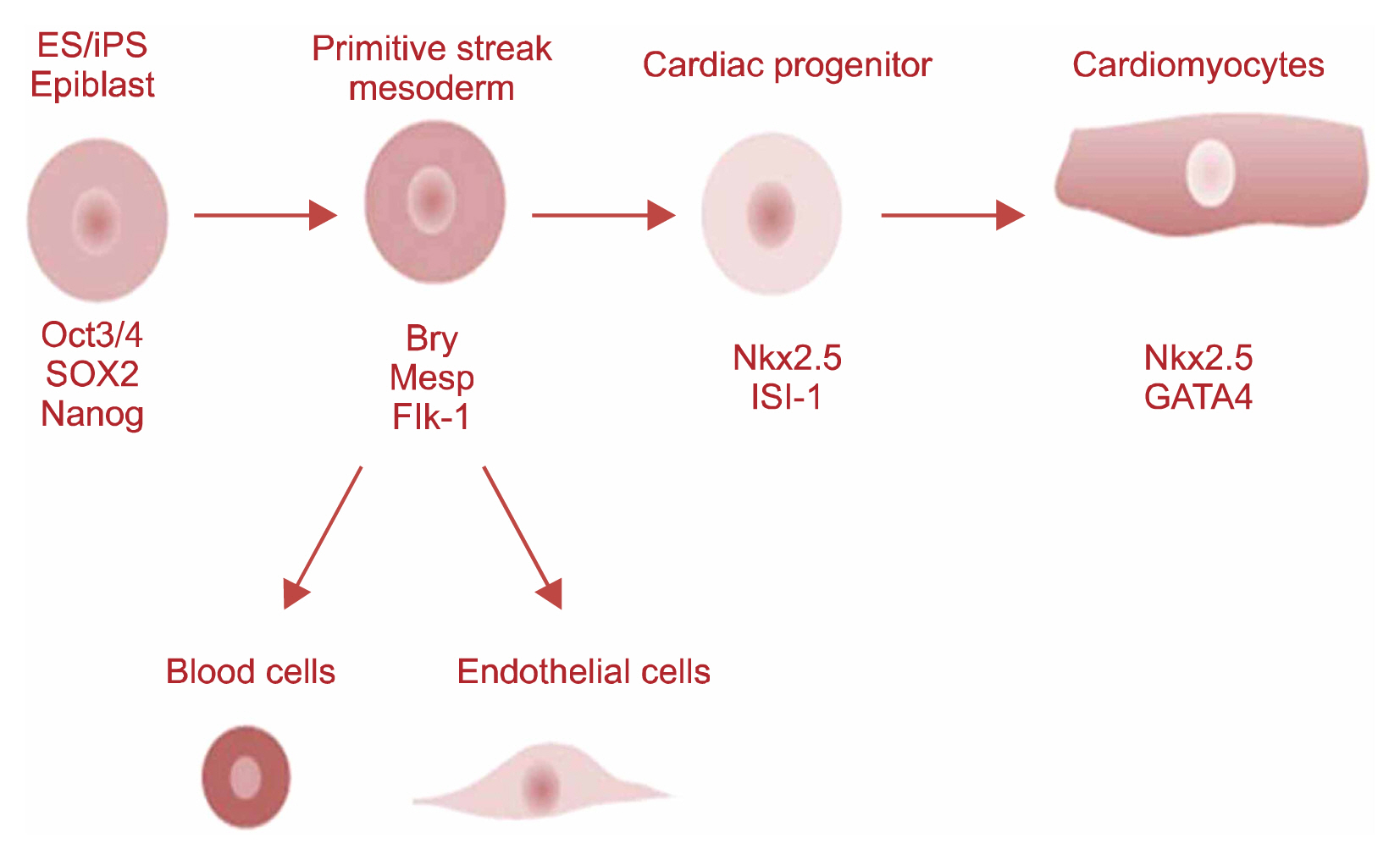
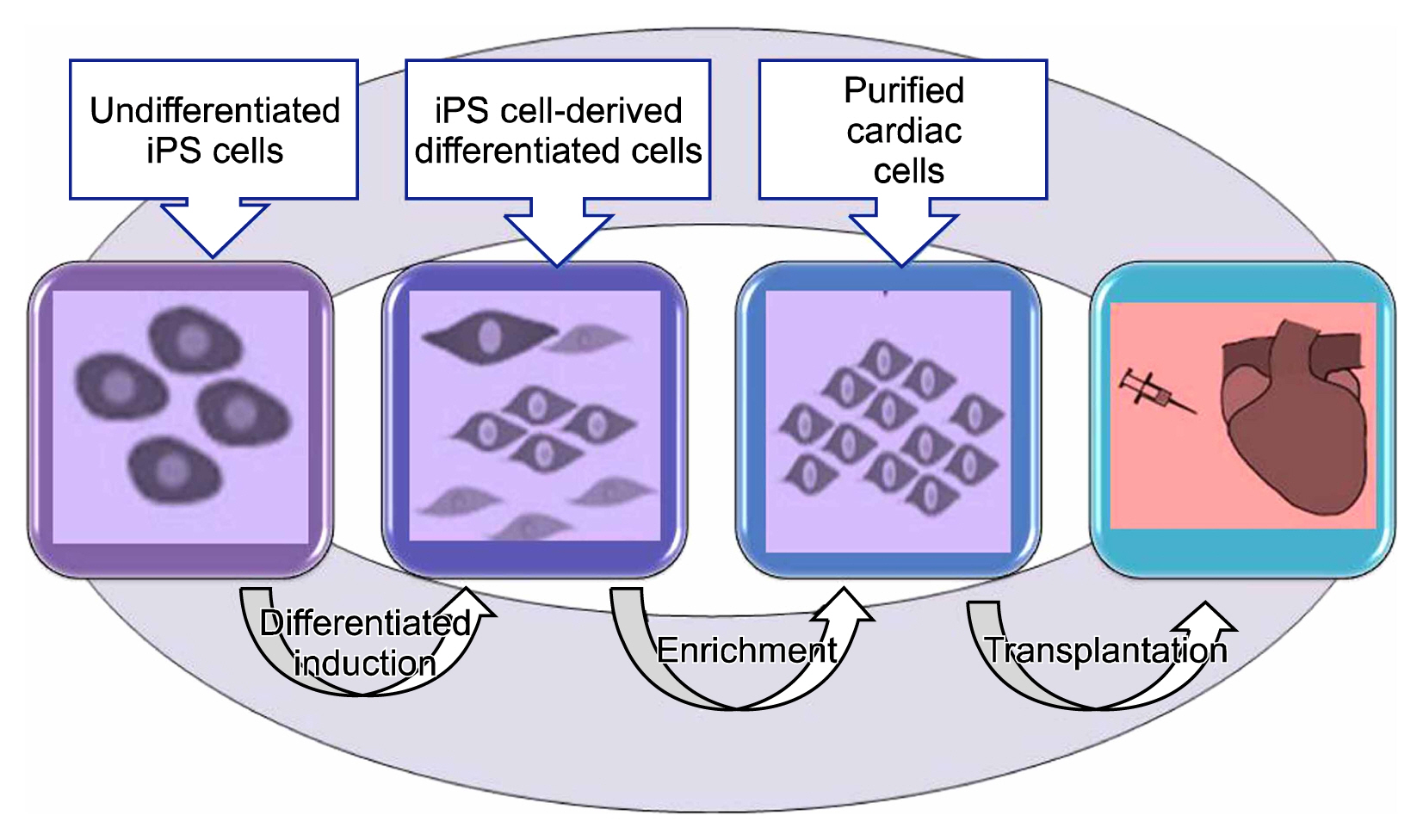
- Stem cells are primitive self renewing undifferentiated cell that can be differentiated into various types of specialized cells like nerve cell, skin cells, muscle cells, intestinal tissue, and blood cells. Stem cells live in bone marrow where they divide to make new blood cells and produces peripheral stem cells in circulation. Under proper environment and in presence of signaling molecules stem cells begin to develop into specialized tissues and organs. These unique characteristics make them very promising entities for regeneration of damaged tissue. Day by day increase in incidence of heart diseases including left ventricular dysfunction, ischemic heart disease (IHD), congestive heart failure (CHF) are the major cause of morbidity and mortality. However infracted tissue cannot regenerate into healthy tissue. Heart transplantation is only the treatment for such patient. Due to limitation of availability of donor for organ transplantation, a focus is made for alternative and effective therapy to treat such condition. In this review we have discussed the new advances in stem cells such as use of cord stem cells and iPSC technology in cardiac repair. Future approach of CB cells was found to be used in tissue repair which is specifically observed for improvement of left ventricular function and myocardial infarction. Here we have also focused on how iPSC technology is used for regeneration of cardiomyocytes and intiating neovascularization in myocardial infarction and also for study of pathophysiology of various degenerative diseases and genetic disease in research field.
Keyword
MeSH Terms
-
Blood Cells
Bone Marrow
Fetal Blood*
Heart Diseases
Heart Failure
Heart Transplantation
Humans
Incidence
Mortality
Muscle Cells
Myocardial Infarction
Myocardial Ischemia
Myocytes, Cardiac
Neurons
Organ Transplantation
Pluripotent Stem Cells*
Regeneration
Skin
Stem Cells*
Tissue Donors
Transplants
Ventricular Dysfunction, Left
Ventricular Function, Left
Figure
Reference
-
References
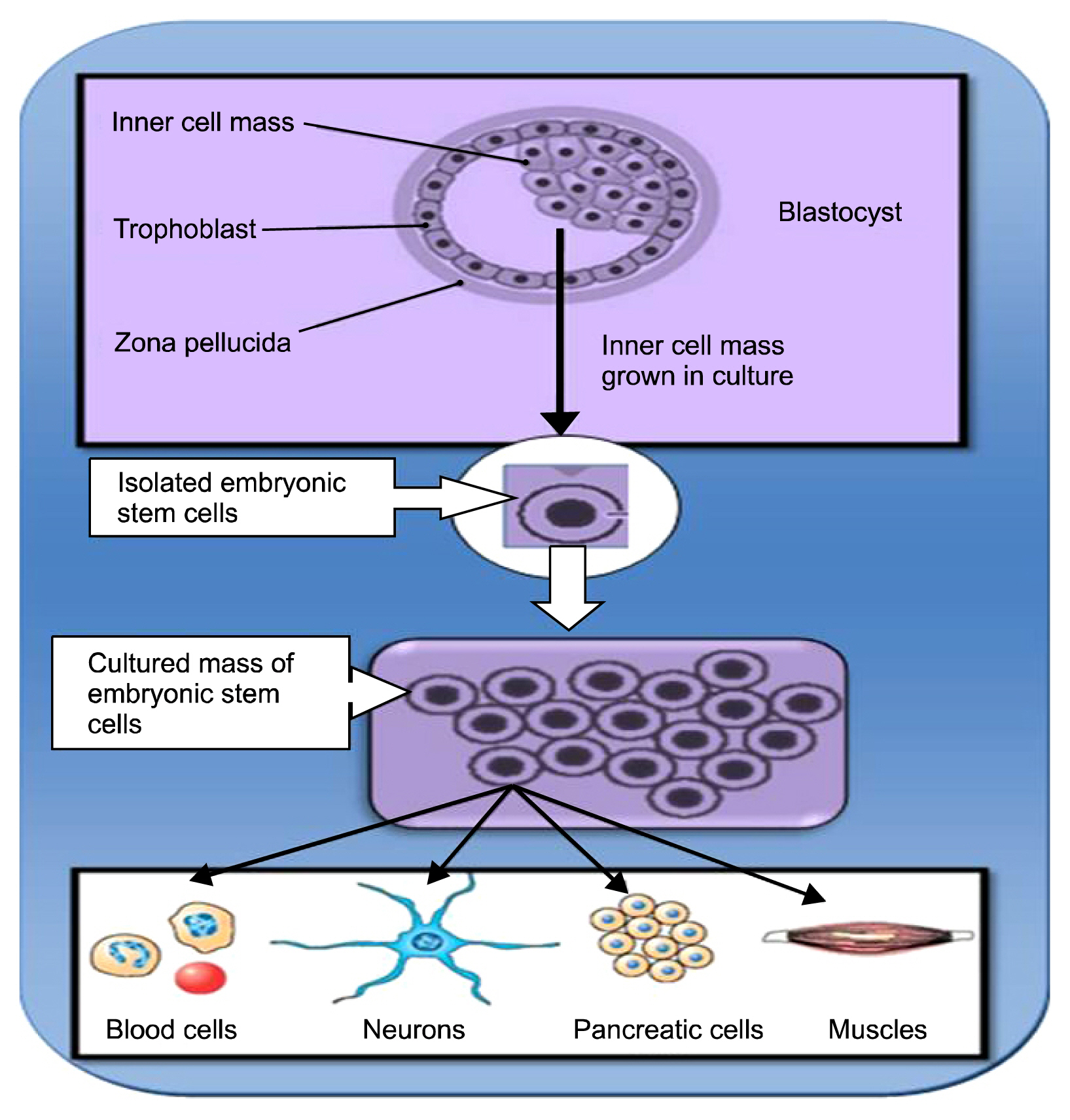
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.
Article2. Zhao Y, Lin B, Darflinger R, Zhang Y, Holterman MJ, Skidgel RA. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS One. 2009; 4:e4226. DOI: 10.1371/journal.pone.0004226. PMID: 19156219. PMCID: 2627485.
Article3. Zhao Y, Mazzone T. Human cord blood stem cells and the journey to a cure for type 1 diabetes. Autoimmun Rev. 2010; 10:103–107. DOI: 10.1016/j.autrev.2010.08.011. PMID: 20728583.
Article4. McKenna D, Sheth J. Umbilical cord blood: Current status & promise for the future. Indian J Med Res. 2011; 134:261–269. PMID: 21985808. PMCID: 3193706.5. What are the potential uses of human stem cells and the obstacles that must be overcome before these potential uses will be realized? Stem Cell Information [World Wide Web site]. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services;2009.6. Kørbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003; 349:570–582. DOI: 10.1056/NEJMra022361. PMID: 12904523.
Article7. Yoshida Y, Yamanaka S. Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation. 2010; 122:80–87. DOI: 10.1161/CIRCULATIONAHA.109.881433. PMID: 20606130.
Article8. Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006; 103:6907–6912. DOI: 10.1073/pnas.0602280103. PMID: 16632596. PMCID: 1458992.
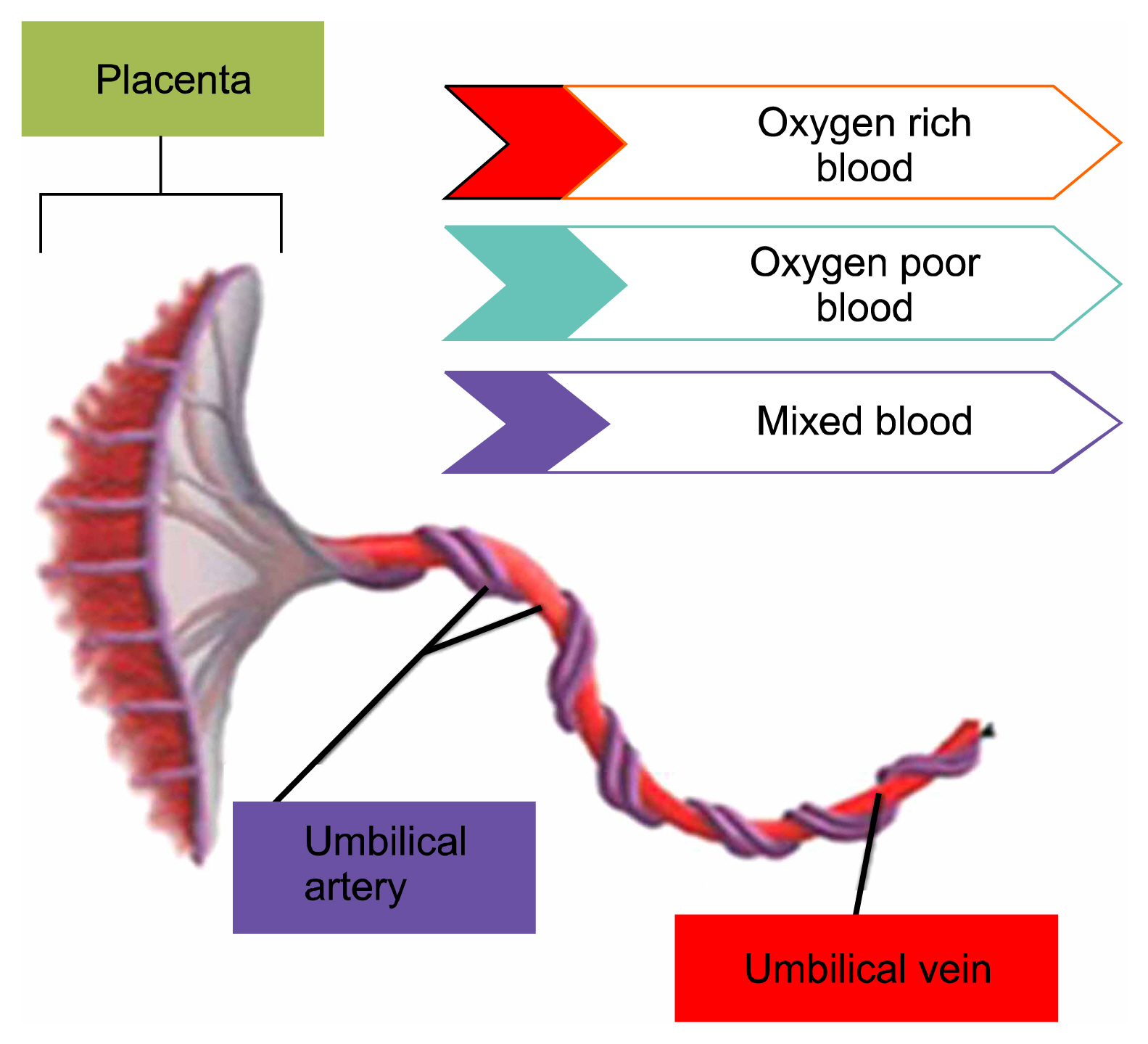
Article9. Moise KJ Jr. Umbilical cord stem cells. Obstet Gynecol. 2005; 106:1393–1407. DOI: 10.1097/01.AOG.0000188388.84901.e4. PMID: 16319269.
Article10. Tortora GJ, Derrickson BH. Development and Inheritance. Roesch B, editor. Principles of Anatomy and Physiology. 12th ed. Chichester: John Wiley & Sons;2009. p. 1144–1145.11. Benirschke K, Kaufmann P. Anatomy and pathology of the umbilical cord and major fetal vessels. Benirschke K, Kaufmann P, editors. Pathology of the human placenta. 4th ed. New York: Springer-Verlag;1995. p. 319–377. DOI: 10.1007/978-1-4757-4196-4_13.
Article12. Hamblin T. Stem cell banking- The growth of public and private cord blood banks. Stem cells, regenerative medicine, and society. World stem cell report. 2009; 168–171.13. Theunissen K, Verfaillie CM. A multifactorial analysis of umbilical cord blood, adult bone marrow and mobilized peripheral blood progenitors using the improved ML-IC assay. Exp Hematol. 2005; 33:165–172. DOI: 10.1016/j.exphem.2004.10.016. PMID: 15676210.
Article14. Mayer H, Bertram H, Lindenmaier W, Korff T, Weber H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005; 95:827–839. DOI: 10.1002/jcb.20462. PMID: 15838884.
Article15. Leor J, Guetta E, Feinberg MS, Galski H, Bar I, Holbova R, Miller L, Zarin P, Castel D, Barbash IM, Nagler A. Human umbilical cord blood-derived CD133+ cells enhance function and repair of the infarcted myocardium. Stem Cells. 2006; 24:772–780. DOI: 10.1634/stemcells.2005-0212.
Article16. Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006; 24:115–124. DOI: 10.1634/stemcells.2005-0053.
Article17. Jeong JA, Gang EJ, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Rapid neural differentiation of human cord blood-derived mesenchymal stem cells. Neuroreport. 2004; 15:1731–1734. DOI: 10.1097/01.wnr.0000134846.79002.5c. PMID: 15257137.
Article18. Kang XQ, Zang WJ, Bao LJ, Li DL, Song TS, Xu XL, Yu XJ. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J Gastroenterol. 2005; 11:7461–7465. DOI: 10.3748/wjg.v11.i47.7391.
Article19. Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005; 330:1153–1161. DOI: 10.1016/j.bbrc.2005.03.086. PMID: 15823564.
Article20. Hutson EL, Boyer S, Genever PG. Rapid isolation, expansion, and differentiation of osteoprogenitors from full-term umbilical cord blood. Tissue Eng. 2005; 11:1407–1420. DOI: 10.1089/ten.2005.11.1407. PMID: 16259596.
Article21. Kadivar M, Khatami S, Mortazavi Y, Shokrgozar MA, Taghikhani M, Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006; 340:639–647. DOI: 10.1016/j.bbrc.2005.12.047.
Article22. Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Müller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004; 200:123–135. DOI: 10.1084/jem.20040440. PMID: 15263023. PMCID: 2212008.
Article23. Wu KH, Zhou B, Yu CT, Cui B, Lu SH, Han ZC, Liu YL. Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Ann Thorac Surg. 2007; 83:1491–1498. DOI: 10.1016/j.athoracsur.2006.10.066. PMID: 17383364.
Article24. Hu CH, Wu GF, Wang XQ, Yang YH, Du ZM, He XH, Xiang P. Transplanted human umbilical cord blood mono-nuclear cells improve left ventricular function through angiogenesis in myocardial infarction. Chin Med J (Engl). 2006; 119:1499–1506.
Article25. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301. DOI: 10.1634/stemcells.2005-0342. PMID: 16410387.
Article26. De Felice L, Tatarelli C, Mascolo MG, Gregorj C, Agostini F, Fiorini R, Gelmetti V, Pascale S, Padula F, Petrucci MT, Arcese W, Nervi C. Histone deacetylase inhibitor valproic acid enhances the cytokine-induced expansion of human hematopoietic stem cells. Cancer Res. 2005; 65:1505–1513. DOI: 10.1158/0008-5472.CAN-04-3063. PMID: 15735039.
Article27. Araki H, Mahmud N, Milhem M, Nunez R, Xu M, Beam CA, Hoffman R. Expansion of human umbilical cord blood SCID-repopulating cells using chromatin-modifying agents. Exp Hematol. 2006; 34:140–149. DOI: 10.1016/j.exphem.2005.10.002. PMID: 16459182.
Article28. Suzuki M, Harashima A, Okochi A, Yamamoto M, Naka-mura S, Motoda R, Yamasaki F, Orita K. 5-Azacytidine supports the long-term repopulating activity of cord blood CD34(+) cells. Am J Hematol. 2004; 77:313–315. DOI: 10.1002/ajh.20178. PMID: 15495241.
Article29. Shyu WC, Lee YJ, Liu DD, Lin SZ, Li H. Homing genes, cell therapy and stroke. Front Biosci. 2006; 11:899–907. DOI: 10.2741/1846.
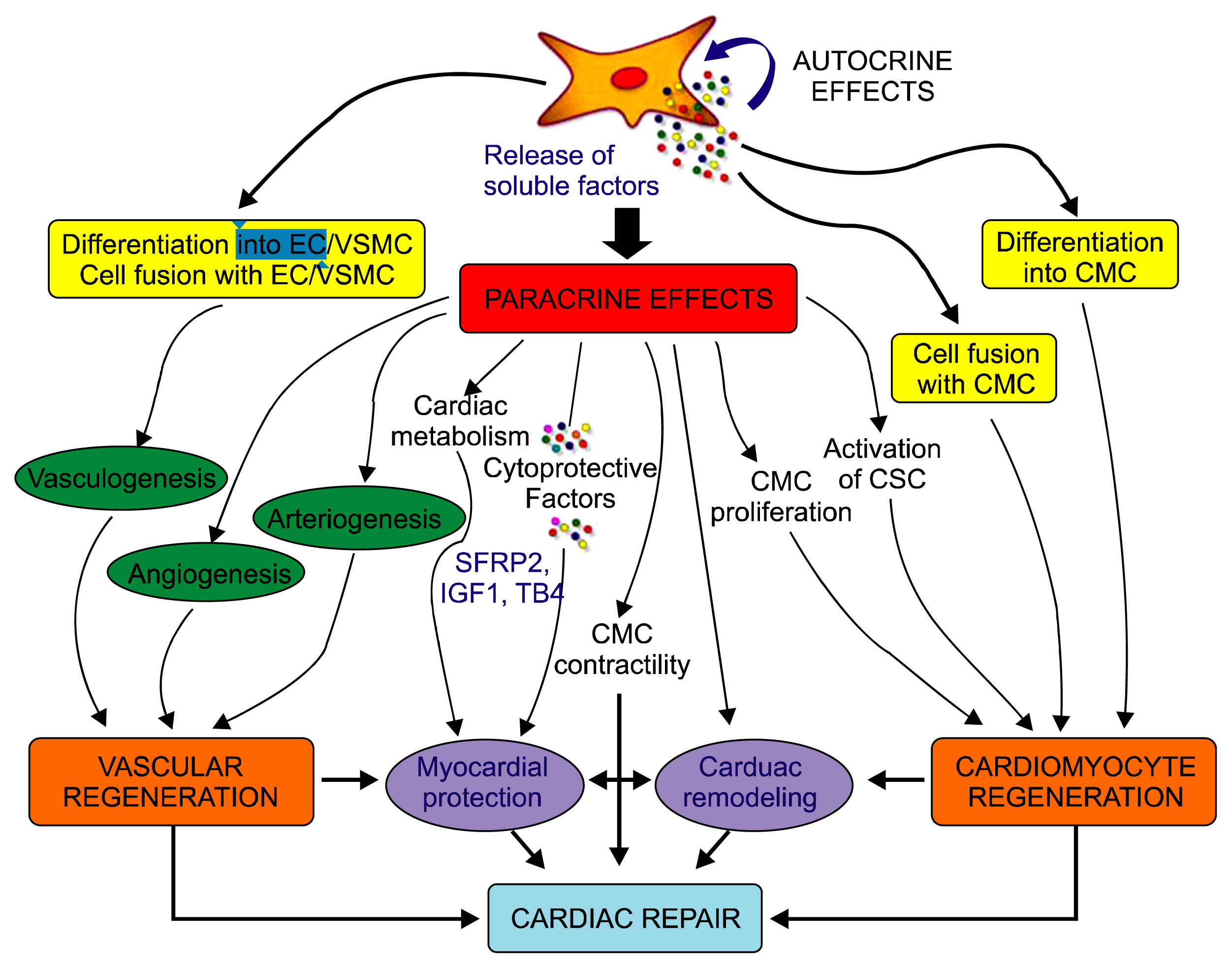
Article30. Gnecchi M, Danieli P, Cervio E. Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol. 2012; 57:48–55. DOI: 10.1016/j.vph.2012.04.002. PMID: 22521741.
Article31. Gnecchi M, Zhang Z, Ni A, Dzau YJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219. DOI: 10.1161/CIRCRESAHA.108.176826. PMID: 19028920. PMCID: 2667788.
Article32. Furfaro EM, Gaballa MA. Do adult stem cells ameliorate the damaged myocardium? Human cord blood as a potential source of stem cells. Curr Vasc Pharmacol. 2007; 5:27–44. DOI: 10.2174/157016107779317170. PMID: 17266611.
Article33. Sunkomat JNE, Goldman S, Harris DT. Cord blood-derived MNCs delivered intracoronary contribute differently to vascularization compared to CD34 + cells in the rat model of acute ischemia. Stem Cells. 2007; (In Press).34. Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999; 103:697–705. DOI: 10.1172/JCI5298. PMID: 10074487. PMCID: 408125.
Article35. Harris DT, Badowski M, Ahmad N, Gaballa MA. The potential of cord blood stem cells for use in regenerative medicine. Expert Opin Biol Ther. 2007; 7:1311–1322. DOI: 10.1517/14712598.7.9.1311. PMID: 17727322.
Article36. Abotalib Z. Importance of cord blood stem cells in regenerative medicine. Saudi Journal of Biological Sciences. Accepted Date: 25 December 2013. 1–33.37. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676. DOI: 10.1016/j.cell.2006.07.024. PMID: 16904174.
Article38. Ohnuki M, Takahashi K, Yamanaka S. Generation and characterization of human induced pluripotent stem cells. Curr Protoc Stem Cell Biol. 2009; Chapter 4(Unit 4A.2):DOI: 10.1002/9780470151808.sc04a02s9. PMID: 19536759.
Article39. Daley GQ, Lensch MW, Jaenisch R, Meissner A, Plath K, Yamanaka S. Broader implications of defining standards for the pluripotency of iPSCs. Cell Stem Cell. 2009; 4:200–201. DOI: 10.1016/j.stem.2009.02.009. PMID: 19265657.
Article40. Ellis J, Bruneau BG, Keller G, Lemischka IR, Nagy A, Rossant J, Srivastava D, Zandstra PW, Stanford WL. Alternative induced pluripotent stem cell characterization criteria for in vitro applications. Cell Stem Cell. 2009; 4:198–199. DOI: 10.1016/j.stem.2009.02.010. PMID: 19265656.
Article41. Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009; 324:797–801. DOI: 10.1126/science.1172482. PMID: 19325077. PMCID: 2758053.
Article42. Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009; 4:472–476. DOI: 10.1016/j.stem.2009.05.005. PMID: 19481515. PMCID: 2705327.
Article43. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.
Article44. Wilmut I. The first direct reprogramming of adult human fibroblasts. Cell Stem Cell. 2007; 1:593–594. DOI: 10.1016/j.stem.2007.11.013.
Article45. Pawani H, Bhartiya D. Pluripotent stem cells for cardiac regeneration: overview of recent advances & emerging trends. Indian J Med Res. 2013; 137:270–282. PMID: 23563370. PMCID: 3657850.46. Zimmermann WH, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006; 12:452–458. DOI: 10.1038/nm1394. PMID: 16582915.
Article47. Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007; 25:1015–1024. DOI: 10.1038/nbt1327. PMID: 17721512.
Article48. Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001; 33:907–921. DOI: 10.1006/jmcc.2001.1367. PMID: 11343414.
Article49. Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008; 118:507–517. DOI: 10.1161/CIRCULATIONAHA.108.778795. PMID: 18625890.
Article50. Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009; 104:e30–e41. DOI: 10.1161/CIRCRESAHA.108.192237. PMID: 19213953. PMCID: 2741334.
Article51. Liu Z, Zhou J, Wang H, Zhao M, Wang C. Current status of induced pluripotent stem cells in cardiac tissue regeneration and engineering. Regen Med Res. 2013; 1:6. DOI: 10.1186/2050-490X-1-6. PMID: 25984325. PMCID: 4376510.
Article52. Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001; 107:1395–1402. DOI: 10.1172/JCI12150. PMID: 11390421. PMCID: 209322.
Article53. Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, et al. Embryonic endothelial progenitor cells expressing a broad range of pro-angiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005; 19:1576–1578. PMID: 16009705.
Article54. Kupatt C, Hinkel R, Lamparter M, von Brühl ML, Pohl T, Horstkotte J, Beck H, Müller S, Delker S, Gildehaus FJ, Büning H, Hatzopoulos AK, Boekstegers P. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidylinositol 3-kinase/AKT kinase. Circulation. 2005; 112(9 Suppl):I117–I122. PMID: 16159802.55. Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005; 11:367–368. DOI: 10.1038/nm0405-367. PMID: 15812508.
Article56. Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006; 98:1414–1421. DOI: 10.1161/01.RES.0000225952.61196.39. PMID: 16690882.
Article57. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219. DOI: 10.1161/CIRCRESAHA.108.176826. PMID: 19028920. PMCID: 2667788.
Article58. Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007; 104:1643–1648. DOI: 10.1073/pnas.0610024104. PMID: 17251350. PMCID: 1785280.
Article59. Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Müller S, Willhauck M, Spitzweg C, Gildehaus FJ, Münzing W, Hannappel E, Bock-Marquette I, DiMaio JM, Hatzopoulos AK, Boekstegers P, Kupatt C. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008; 117:2232–2240. DOI: 10.1161/CIRCULATIONAHA.107.758904. PMID: 18427126. PMCID: 2672916.
Article60. Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A. 2008; 105:18366–18371. DOI: 10.1073/pnas.0803437105. PMID: 19017790. PMCID: 2587631.
Article61. Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: the Rubik’s cube of cell therapy for heart disease. Dis Model Mech. 2009; 2:344–358. DOI: 10.1242/dmm.000240. PMID: 19553696. PMCID: 2707103.
Article62. Kong CW, Akar FG, Li RA. Translational potential of human embryonic and induced pluripotent stem cells for myocardial repair: insights from experimental models. Thromb Haemost. 2010; 104:30–38. DOI: 10.1160/TH10-03-0189. PMID: 20539906.
Article63. Qian L, Srivastava D. Monkeying around with cardiac progenitors: hope for the future. J Clin Invest. 2010; 120:1034–1036. DOI: 10.1172/JCI42643. PMID: 20335651. PMCID: 2846073.
Article64. Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006; 441:1097–1099. DOI: 10.1038/nature04961. PMID: 16810246.
Article65. Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008; 134:877–886. DOI: 10.1016/j.cell.2008.07.041. PMID: 18691744. PMCID: 2633781.
Article66. Ahmed RP, Ashraf M, Buccini S, Shujia J, Haider HKh. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen Med. 2011; 6:171–178. DOI: 10.2217/rme.10.103. PMID: 21391851. PMCID: 3110348.
Article67. Zhang Y, Wang D, Chen M, Yang B, Zhang F, Cao K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS One. 2011; 6:e19012. DOI: 10.1371/journal.pone.0019012. PMID: 21552563. PMCID: 3084251.
Article