Pediatr Infect Vaccine.
2016 Apr;23(1):54-61. 10.14776/piv.2016.23.1.54.
The Incidence Rate of Lymphadenitis after Bacille Calmette-Guérin (BCG) Vaccination
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, The Catholic University of Korea, Seoul, Korea. jh00mn@catholic.ac.kr
- 2Department of Pediatrics, Sungsae Hospital, Pyeongtaek, Korea.
- 3Dr. Ha Jeong Hun's Pediatric Clinic, Seoul, Korea.
- 4Hanmaeum Pediatric Clinic, Suwon, Korea.
- KMID: 2163224
- DOI: http://doi.org/10.14776/piv.2016.23.1.54
Abstract
- PURPOSE
Bacille Calmette-Guérin (BCG) lymphadenitis is a relatively frequent local adverse reactions after BCG vaccination. Its incidence rate is usually <1%. However, this rate may be different according to BCG strain, vaccination method or skill, etc. In the Republic of Korea, two BCG strains are used: intradermal Danish-1331 or percutaneous Tokyo-172. We surveyed the incidence rates of BCG lymphadenitis.
METHODS
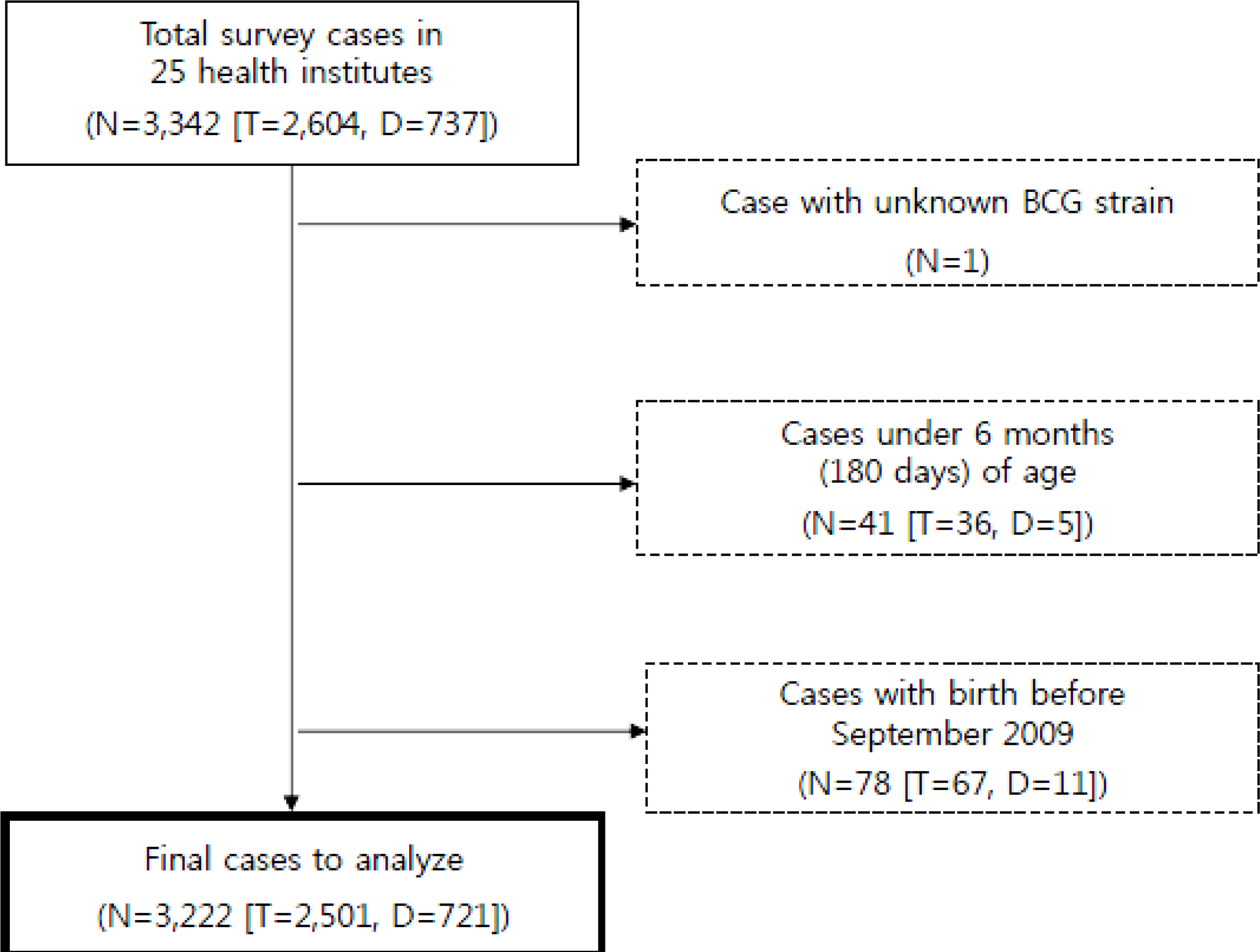
This survey was performed in total 25 centers (5 general hospitals, 20 private pediatric clinics). Immunized type of BCG strain in study subjects was verified by directly observing the scar. The occurrence of BCG lymphadenitis was asked to their parent. In cases of BCG lymphadenitis, location, diameter size, progression of suppuration, and treatment method were investigated, as well.
RESULTS
The total number of study subjects was 3,342. Among these, the subjects suitable for enrollment criteria (total 3,222; Tokyo strain 2,501, Danish strain 721) were analyzed. BCG lymphadenitis regardless of its size developed in each five of subjects per strains, therefore, its incidence rate was 0.20% in Tokyo and 0.69% in Danish strain, respectively (P=0.086). However, when applying the WHO criteria - the development of lymph node swelling with diameter 1.5 cm or more, the incidence rate of BCG lymphadenitis was 0.16% (4 cases) in Tokyo and 0.42% (3 cases) in Danish strain, respectively.
CONCLUSIONS
The incidence rate of lymphadenitis in two BCG types, percutaneous Tokyo and intradermal Danish strain BCG, is 0.20% and 0.69%, respectively. Both rates are acceptable.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. BCG vaccine: WHO position paper. Wkly Epidemiol Rec. 2004; 79:27–38.2. Milstien IB, Gibson II. Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ. 1990; 68:93–108.3. The Korean Pediatric Society. BCG vaccine. Lee H], editor. Immunization guideline. 7th ed.Seoul: The Korean Pediatric Society;2012. p. 40–54.4. Lotte A, Wasz-Hockert O, Poisson N, Dumitrescu N, Verron M, Couvet E. A bibliography of the complications of BCG vaccination. A comprehensive list of the world literature since the introduction of BCG up to July 1982, supplemented by over 100 personal communications. Adv Tuberc Res. 1984; 21:194–245.5. World Health Organization. Immunization safety surveillance. Guidelines for managers of immunization programmes on reporting and investigating adverse events following immunization. Manila: Western Pacific Regional Office, WHO. 1999.6. Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille Calmette—Guérin disease after vaccination: case report and review. Clin Infect Dis 19972411. 139–46.7. Ustvedt HI. Local reactions in BCG vaccination. Bull World Health Organ. 1950; 2:441–68.8. Goraya IS, Virdi VS. Bacille Calmette-Guérin lymphadenitis. Postgrad Med I. 2002; 78:327–9.9. Mori T, Yamauchi Y, Shiozawa K. Lymph node swelling due to bacille Camette-Guerin vaccination with multipuncture method. Tuber Lung Dis. 1996; 77:269–3.10. Daoud W. Control of an outbreak of BCG complications in Gaza. Respirology. 2003; 8:376–8.
Article11. Jeena PM, Chhagan MK, Topley I, Coovadia HM. Safety of the intradermal Copenhagen 1331 BCG vaccine in neonates in Durban, South Africa. Bull World Health Organ. 2001; 79:337–43.12. R Core Team (2015). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at:. https://WWW.R-project. org/. Assessed 26 October. 2015.13. Corbel M]. Fruth U, Griffiths E, Knezevic I. Report on a WHO consultation on the characterisation of BCG strains, Imperial College, London 15-16 December 2003. Vaccine. 2004; 22:2675–80.
Article14. Smith KC, Orme IM, Starke IR. Tuberculosis vaccines. Plotkin SA, Orenstein WA, Oflit PA, editors. editors.Vaccines. 6th ed.Philadelphia: Elsevier Inc;2006. p. 789–81. 1.
Article15. Fine PEM, Carneiro IAM, Milstien IB, Clements CI. Issues relating to the use of BCG in immunization programmes: a discussion document. Geneva: WHO;1999.16. Samileh N, Ahmad S, Farzaneh A, Shahnaz R, Lida F, Mo-hammad N. Immunity status in children With Bacille Ca1-mette—Guerin adenitis. A prospective study in Tehran, Iran. Saudi Med I. 2006; 27:1719–24.17. Serour F, Mizrahi A, Somekh E, Feinberg I, Picard C, Casanova IL, et al. Analysis of the interleukin-12/interferon-gamma pathway in children with non-tuberculous mycobacterial cervical lymphadenitis. Eur J Pediatr. 2007; 166:835–41.18. Yeganeh M, Heidarzade M, Pourpak Z, Parvaneh N, Rezaei N, Gharagozlou M, et al. Severe combined immunodefici-ency: a cohort of 40 patients. Pediatr Allergy Immunol. 2008; 19:303–6.
Article19. Lee PP, Chan KW Jiang L, Chen T, Li C, Lee TL, et al. Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr Infect Dis I. 2008; 27:224–30.20. Nyerges G, Drinoczy M. Significance of the number of Viable units in BCG vaccines. Dev Biol Stand. 1986; 58:331 -6.21. Teulieres L, Diouf MA, Chaud P, Saint-Cyr A, Saliou P. Comparative trial of administration of half (0.05 mg) and quarter (0.025 mg) dose of intradermal Pasteur BCG on 291 infants from birth to 1 year in French Guyana. Vaccine. 1991; 9:521–4.
Article22. Tee SS, Smeulders N, Shingadia DV. BCG vaccine-associated suppurative lymphadenitis. Vaccine. 2005; 23:2676–9.
Article23. Gheorghiu M. The present and future role of BCG vaccine in tuberculosis control. Biologicals. 1990; 18:135–41.
Article24. Bolger T, O'Connell M, Menon A, Butler K. Complications associated with the bacille Calmette-Guerin vaccination in Ireland. Arch Dis Child. 2006; 91:594–7.
Article25. World Health Organization. Expanded programme on immunization, biologicals unit: lymphadenitis associated with BCG immunization. Wkly Epidemiol Rec. 1988; 63:381–8.26. Baek HS, Chang JY, Moon SJ, Oh SH. Lymphadenitis following intradermal BCG vaccination. Korean I Pediatr. 2006; 49:46–50.
Article27. Hwang IS, Choi YY, Ma IS, Hwang T]. A clinical study on BCG lymphadenitis. Korean I Pediatr. 1997; 40:614–8.28. Lee IS, Sohn YM. Observation of response to PPD skin test and local side reactions at multiple inoculation sites after percutaneous inoculation with BCG Tokyo 172 strain. Korean J Pediatr Infect Dis. 2000. 72201–10.29. Oh MH, Kim KH, Sim JG. The clinical study on conversion rate of Mantoux test, change of local lesion and complication after multipuncture BCG vaccination in neonates. Korean I Pediatr. 1997; 40:1120–30.30. Kim HJ, Oh SY, Lee IB. Comparison of each strains (Pasteur, Danish, Tokyo) of BCG efficacy using tuberculin test and adverse reactions. Korea Center for Diseases Control and Prevention, Osong. 2008.31. Goraya IS, Virdi VS. Treatment of Calmette-Guerin Bacillus adenitis; a metaanalysis. Pediatr Infect Dis I. 2001; 20:632–4.32. Banani SA, Alborzi A. Neddle aspiration for suppurative post—BCG adenitis. Arch Dis Child. 1994; 71:446–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant Bacille Calmette–Guérin for Immunotherapy in Nonmuscle Invasive Bladder Cancer
- Clinical Significance of the Bacille Calmette-Guérin Site Reaction in Kawasaki Disease Patients Aged Less than 18 Months
- Supraclavicular BCG Lymphadenitis Noted at 21 Months after BCG Vaccination Confirmed by a Molecular Method
- Management of BCG lymphadenitis
- Mycobacterium bovis Osteitis Following Immunization with Bacille Calmette-Guérin (BCG) in Korea