J Cardiovasc Ultrasound.
2016 Mar;24(1):60-63. 10.4250/jcu.2016.24.1.60.
Heart within a Heart
- Affiliations
-
- 1Department of Internal Medicine, Henry Ford Hospital, Detroit, MI, USA. tarun93@hotmail.com
- 2Department of Cardiology, Henry Ford Hospital, Detroit, MI, USA.
- KMID: 2160997
- DOI: http://doi.org/10.4250/jcu.2016.24.1.60
Abstract
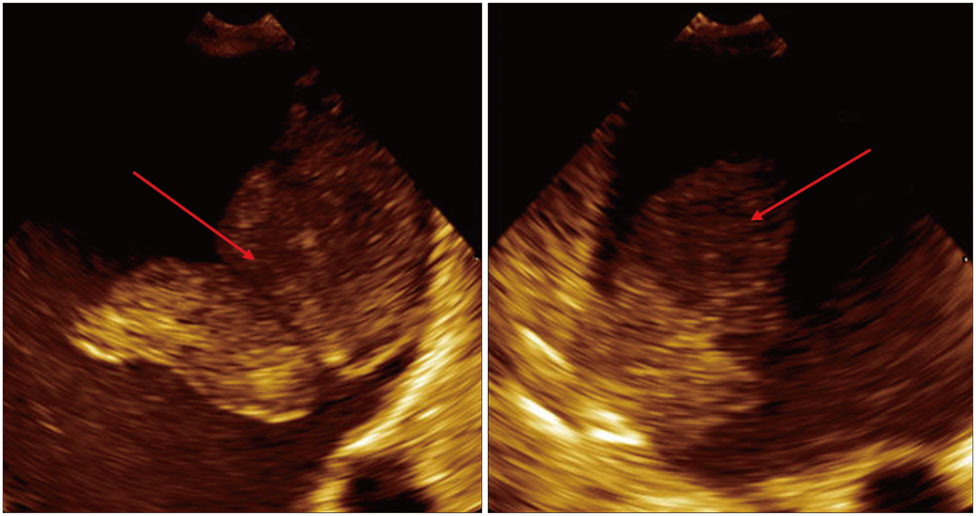
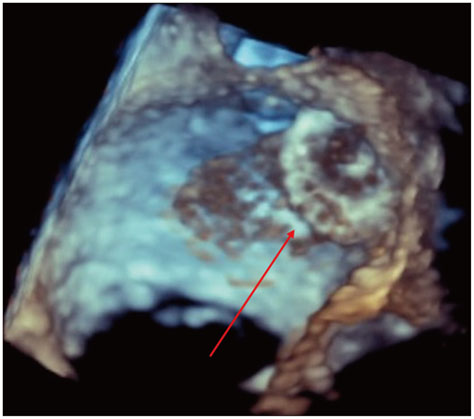
- Device based closure of the left atrial appendage (LAA) has emerged as a viable approach for stroke prevention in atrial fibrillation (AF) patients with contraindications to chronic oral anticoagulation. One of the most feared complications is device related thrombus formation. We present a 66-year-old male with chronic AF who developed a life-threatening intracranial bleed on oral anti-coagulation. He subsequently underwent LAA closure using an Amplatzer muscular ventricular septal defect closure device for stroke prevention. However, he was found to have a large thrombus attached to the device a year later. We present a review of the various LAA closure devices, importance of periodic surveillance via echocardiography and management options to prevent this complication. Also, the case highlights the importance of contrast-enhance echocardiography in diagnosis of LAA closure device thrombus.
MeSH Terms
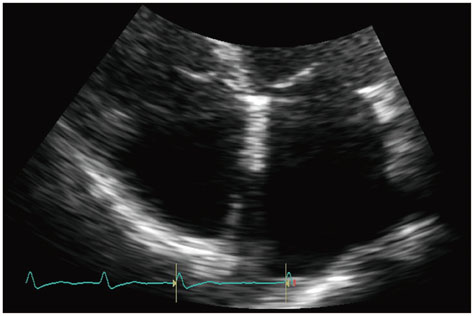
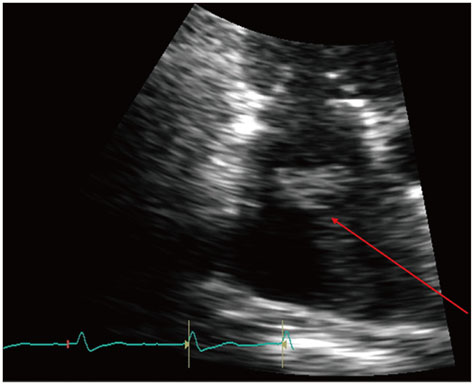
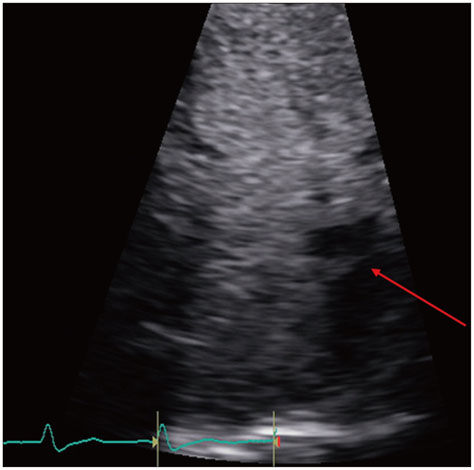
Figure
Reference
-
1. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011; 123:e18–e209.2. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996; 61:755–759.3. Lardizabal JA, Sharma S. Mechanical approaches to stroke prevention in atrial arrhythmias. J Innov Cardiac Rhythm Manage. 2012; 3:809–813.4. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009; 374:534–542.5. Reddy VY, Möbius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, Sick P, Sievert H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013; 61:2551–2556.6. De Backer O, Arnous S, Ihlemann N, Vejlstrup N, Jørgensen E, Pehrson S, Krieger TD, Meier P, Søndergaard L, Franzen OW. Percutaneous left atrial appendage occlusion for stroke prevention in atrial fibrillation: an update. Open Heart. 2014; 1:e000020.7. Plicht B, Konorza TF, Kahlert P, Al-Rashid F, Kaelsch H, Jánosi RA, Buck T, Bachmann HS, Siffert W, Heusch G, Erbel R. Risk factors for thrombus formation on the Amplatzer Cardiac Plug after left atrial appendage occlusion. JACC Cardiovasc Interv. 2013; 6:606–613.8. Urena M, Rodés-Cabau J, Freixa X, Saw J, Webb JG, Freeman M, Horlick E, Osten M, Chan A, Marquis JF, Champagne J, Ibrahim R. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013; 62:96–102.9. Cardona L, Ana G, Luísa B, Leal A, António F, Lídia S, Cruz FR. Thrombus formation on a left atrial appendage closure device. Circulation. 2011; 124:1595–1596.10. Lammers J, Elenbaas T, Meijer A. Thrombus formation on an Amplatzer closure device after left atrial appendage closure. Eur Heart J. 2013; 34:741.11. Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig SM, Burns PN, Castello R, Coon PD, Hagen ME, Jollis JG, Kimball TR, Kitzman DW, Kronzon I, Labovitz AJ, Lang RM, Mathew J, Moir WS, Nagueh SF, Pearlman AS, Perez JE, Porter TR, Rosenbloom J, Strachan GM, Thanigaraj S, Wei K, Woo A, Yu EH, Zoghbi WA. American Society of Echocardiography. American society of echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr. 2008; 21:1179–1201. quiz 1281
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Congenital heart disease with left to right shunt
- Key Role of the Korean Society of Heart Failure: Moving Towards a Global and Individualized Approach
- The Korean Society of Heart Failure: Breaking Barriers, Bridging Solutions Together!
- A Practical Way to Reduce Healthcare Costs in Patients With Heart Failure: Outpatient IV Diuretics Therapy
- Clinical study for reoperations on heart valve diseases