J Gynecol Oncol.
2015 Apr;26(2):134-140. 10.3802/jgo.2015.26.2.134.
Prognostic impact of lymphadenectomy in uterine clear cell carcinoma
- Affiliations
-
- 1Division of Gynecologic Oncology, Ob/Gyn & Women's Health Institute, Cleveland Clinic, Cleveland, OH, USA. mahdih6281@gmail.com
- 2Department of Biostatistics, University of Washington, Seattle, WA, USA.
- KMID: 2160796
- DOI: http://doi.org/10.3802/jgo.2015.26.2.134
Abstract
OBJECTIVE
The aim of this study was to estimate the survival impact of lymphadenectomy in patients diagnosed with uterine clear cell cancer (UCCC).
METHODS
Patients with a diagnosis of UCCC were identified from Surveillance, Epidemiology, and End Results (SEER) program from 1988 to 2007. Only surgically treated patients were included. Statistical analysis using Student t-test, Kaplan-Meier survival methods, and Cox proportional hazard regression were performed.
RESULTS
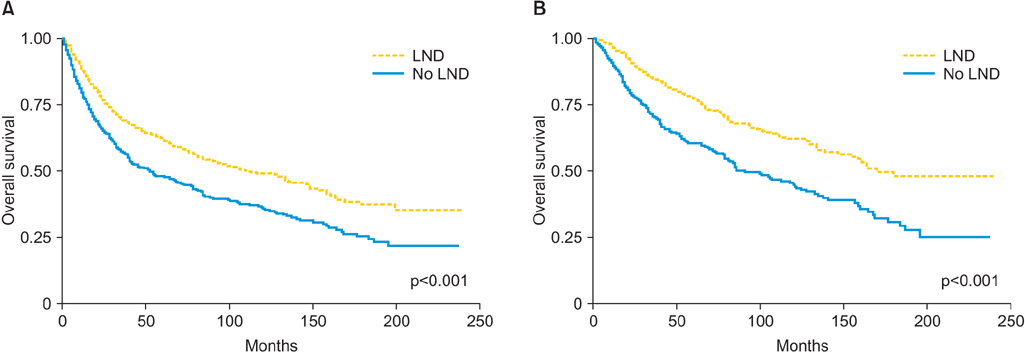
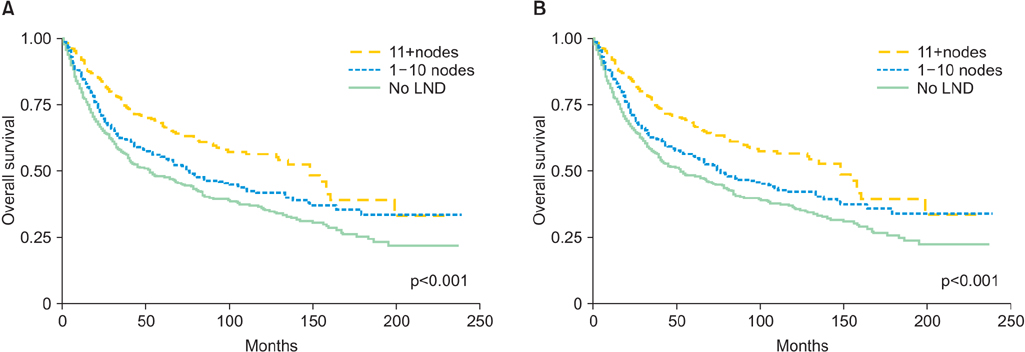
One thousand three hundred eighty-five patients met the inclusion criteria; 955 patients (68.9%) underwent lymphadenectomy. Older patients (> or =65) were less likely to undergo lymphadenectomy compared with their younger cohorts (64.3% vs. 75.9%, p<0.001). The prevalence of nodal metastasis was 24.8%. Out of 724 women who had disease clinically confined to the uterus and underwent lymphadenectomy, 123 (17%) were found to have nodal metastasis. Lymphadenectomy was associated with improved survival. Patients who underwent lymphadenectomy were 39% (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.52 to 0.72; p<0.001) less likely to die than patient who did not have the procedure. Moreover, more extensive lymphadenectomy correlated positively with survival. Compared to patients with 0 nodes removed, patients with more extensive lymphadenectomy (1 to 10 and >10 nodes removed) were 32% (HR, 0.68; 95% CI, 0.56 to 0.83; p<0.001) and 47% (HR, 0.53; 95% CI, 0.43 to 0.65; p<0.001) less likely to die, respectively.
CONCLUSION
The extent of lymphadenectomy is associated with an improved survival of patients diagnosed with UCCC.
MeSH Terms
-
Adenocarcinoma, Clear Cell/*diagnosis/mortality/pathology/*surgery
Adult
Aged
Aged, 80 and over
Endometrial Neoplasms/*diagnosis/mortality/pathology/*surgery
Female
Humans
*Lymph Node Excision
Lymphatic Metastasis
Middle Aged
Pelvis
Prognosis
Retrospective Studies
Survival Rate
Uterine Neoplasms/diagnosis/mortality/pathology/surgery
Figure
Reference
-
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29.2. Silverberg SG, De Giorgi LS. Clear cell carcinoma of the endometrium. Clinical, pathologic, and ultrastructural findings. Cancer. 1973; 31:1127–1140.3. Kurman RJ, Scully RE. Clear cell carcinoma of the endometrium: an analysis of 21 cases. Cancer. 1976; 37:872–882.4. Kay S. Clear-cell carcinoma of the endometrium. Cancer. 1957; 10:124–130.5. Clement PB, Young RH. Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2004; 11:117–142.6. Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006; 94:642–646.7. Cirisano FD Jr, Robboy SJ, Dodge RK, Bentley RC, Krigman HR, Synan IS, et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecol Oncol. 1999; 74:385–394.8. Christopherson WM, Alberhasky RC, Connelly PJ. Carcinoma of the endometrium. I. A clinicopathologic study of clear-cell carcinoma and secretory carcinoma. Cancer. 1982; 49:1511–1523.9. Abeler VM, Vergote IB, Kjorstad KE, Trope CG. Clear cell carcinoma of the endometrium. Prognosis and metastatic pattern. Cancer. 1996; 78:1740–1747.10. Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004; 95:593–596.11. Matthews RP, Hutchinson-Colas J, Maiman M, Fruchter RG, Gates EJ, Gibbon D, et al. Papillary serous and clear cell type lead to poor prognosis of endometrial carcinoma in black women. Gynecol Oncol. 1997; 65:206–212.12. Carcangiu ML, Chambers JT. Early pathologic stage clear cell carcinoma and uterine papillary serous carcinoma of the endometrium: comparison of clinicopathologic features and survival. Int J Gynecol Pathol. 1995; 14:30–38.13. Thomas M, Mariani A, Wright JD, Madarek EO, Powell MA, Mutch DG, et al. Surgical management and adjuvant therapy for patients with uterine clear cell carcinoma: a multi-institutional review. Gynecol Oncol. 2008; 108:293–297.14. DuBeshter B, Estler K, Altobelli K, McDonald S, Glantz C, Angel C. High-dose rate brachytherapy for Stage I/II papillary serous or clear cell endometrial cancer. Gynecol Oncol. 2004; 94:383–386.15. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2008 Sub (1973-2006 varying)-Linked To County Attributes-Total U.S., 1969-2006 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009, based on the November 2008 submission [Internet]. Bethesda, MD: National Cancer Institute;2014. cited 2015 Feb 3. Available from: http://www.seer.cancer.gov.16. Cirisano FD Jr, Robboy SJ, Dodge RK, Bentley RC, Krigman HR, Synan IS, et al. The outcome of stage I-II clinically and surgically staged papillary serous and clear cell endometrial cancers when compared with endometrioid carcinoma. Gynecol Oncol. 2000; 77:55–65.17. Ahmed A, Zamba G, DeGeest K, Lynch CF. The impact of surgery on survival of elderly women with endometrial cancer in the SEER program from 1992-2002. Gynecol Oncol. 2008; 111:35–40.18. Wright JD, Lewin SN, Barrena Medel NI, Sun X, Burke WM, Deutsch I, et al. Endometrial cancer in the oldest old: tumor characteristics, patterns of care, and outcome. Gynecol Oncol. 2011; 122:69–74.19. Maxwell GL, Tian C, Risinger J, Brown CL, Rose GS, Thigpen JT, et al. Racial disparity in survival among patients with advanced/recurrent endometrial adenocarcinoma: a Gynecologic Oncology Group study. Cancer. 2006; 107:2197–2205.20. Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009; 115:1276–1285.21. Krag DN, Single RM. Breast cancer survival according to number of nodes removed. Ann Surg Oncol. 2003; 10:1152–1159.22. Tepper JE, O'Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB 3rd, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001; 19:157–163.23. Shah M, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ, et al. Therapeutic role of lymphadenectomy for cervical cancer. Cancer. 2011; 117:310–317.24. Cragun JM, Havrilesky LJ, Calingaert B, Synan I, Secord AA, Soper JT, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005; 23:3668–3675.25. Chan JK, Cheung MK, Huh WK, Osann K, Husain A, Teng NN, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006; 107:1823–1830.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of survival between radical nephrectomy with and without extensive lyphadenectomy in renal cell racinoma
- A case of clear cell carcinoma that is unrelated to diethystilbestrol of the uterine cervix
- Clear Cell Carcinoma Presented as a Large Polypoid Mass Expanding the Vaginal Fornix: Report of Two Cases
- Surgical management of non-invasive uterine clear cell carcinoma
- The Impact of Pelvic Lymphadenectomy on the Survival of Patients Who Underwent Radical Cystectomy for Transitional Cell Carcinoma of the Bladder