Yonsei Med J.
2007 Aug;48(4):634-638. 10.3349/ymj.2007.48.4.634.
Association between Internal Carotid Artery Morphometry and Posterior Communicating Artery Aneurysm
- Affiliations
-
- 1Department of Neurosurgery, School of Medicine, Wonkwang University, Iksan, Korea. kangsd@wonkwang.ac.kr
- KMID: 2158168
- DOI: http://doi.org/10.3349/ymj.2007.48.4.634
Abstract
- PURPOSE
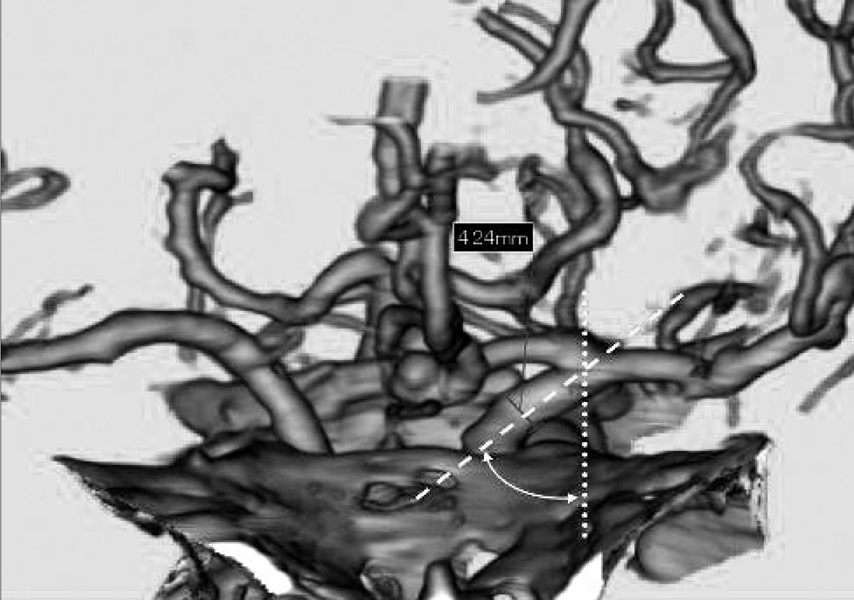
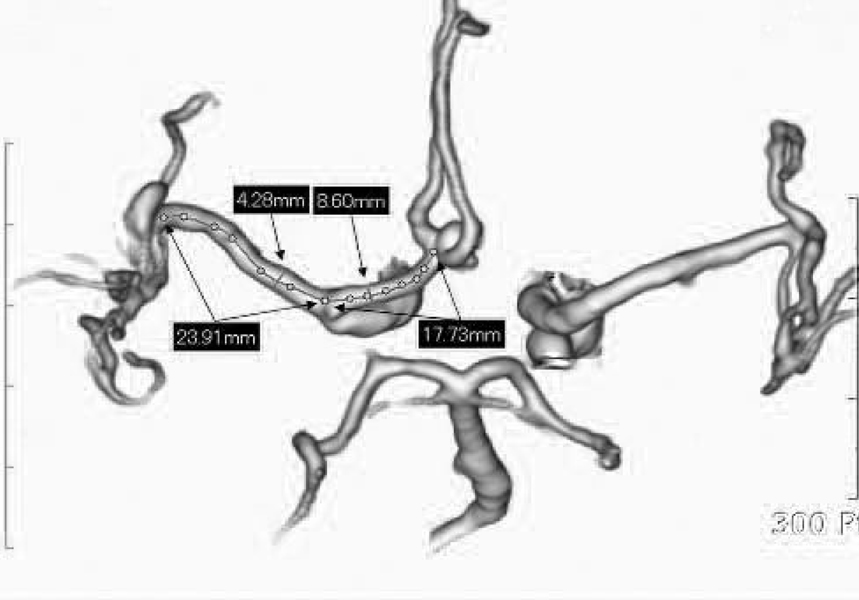
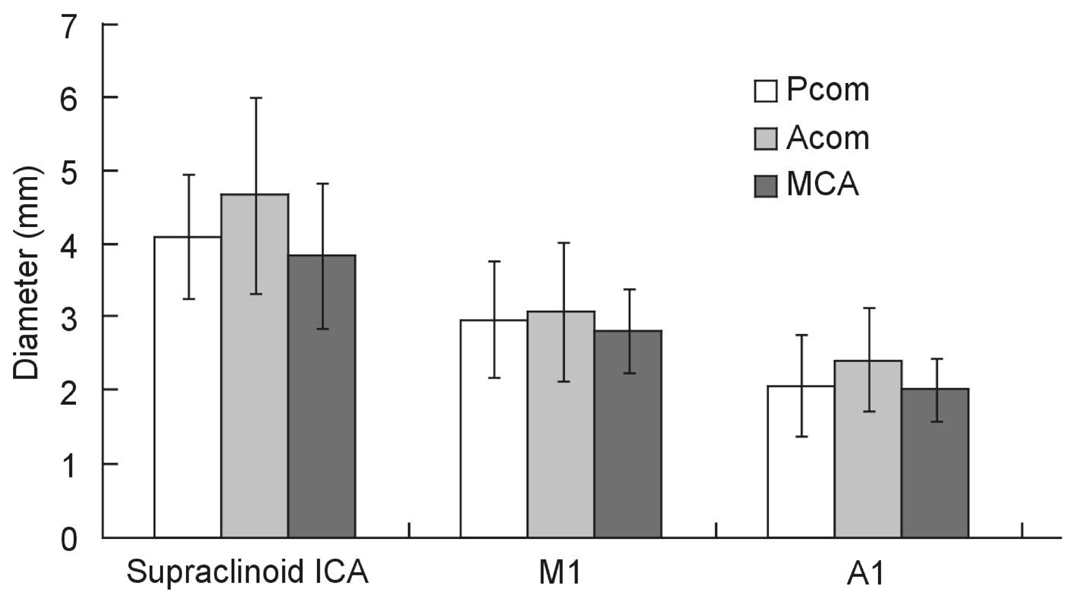
The goal of this study was to directly measure the association between the internal carotid artery (ICA) morphometry and the presence of ICA-posterior communicating artery (PCOM) aneurysm. MATERIALS AND METHODS: The authors intraoperatively measured the length of the supraclinoid ICA because it is impossible to radiologically determine the exact location of the anterior clinoid process. We used an image analyzer with a CT angiogram to measure the angle between the skull midline and the terminal segment of the ICA (ICA angle), as well as the diameter of the ICA. The lengths and diameters of the supraclinoid ICA and the ICA angle were compared among PCOM aneurysms, anterior communicating artery (ACOM) aneurysms, and middle cerebral artery (MCA) bifurcation aneurysms (n=27 each). Additionally, the lengths and the diameters of M1 and A1 were compared for each aneurysm. RESULTS: The lengths of the supraclinoid ICA were 11.9+/-2.3mm. The lengths of the supraclinoid ICA in patients with ICA-PCOM aneurysms (9.7+/-2.8mm) were shorter than those of patients with ACOM aneurysms (13.8+/-2.2mm, Student's t-test, p<0.001) and with MCA bifurcation aneurysms (12.2+/-1.9 mm, Student's t-test, p<0.001). The diameters of the supraclinoid ICA and A1 in patients with ACOM aneurysms were larger than those in patients with MCA bifurcation aneurysms (Student's t-test, p<0.05). There were no significant differences in the lengths of M1 and A1, ICA angle, or diameter of M1 for each aneurysm. CONCLUSION: These results suggest that the relatively shorter length of the supraclinoid ICA may be a novel risk factor for the development of ICA-PCOM aneurysm with higher hemodynamic stress.
MeSH Terms
Figure
Cited by 1 articles
-
The Usefulness of Extradural Anterior Clinoidectomy for Low-Lying Posterior Communicating Artery Aneurysms : A Cadaveric Study
Hyoung Soo Byoun, Kyu-Sun Choi, Min Kyun Na, Sae Min Kwon, Yong Seok Nam
J Korean Neurosurg Soc. 2024;67(4):411-417. doi: 10.3340/jkns.2023.0184.
Reference
-
1. Evans JJ, Hwang YS, Lee JH. Pre- versus post-anterior clinoidectomy measurements of the optic nerve, internal carotid artery, and opticocarotid triangle: a cadaveric morphometric study. Neurosrugery. 2000. 46:1018–1023.
Article2. Gibo H, Lenkey C, Rhoton AL. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981. 55:560–574.
Article3. Marinković S, Kovacević M, Millisavljević M. Hypplasia of the proximal segment of the anterior cerebral artery. Anat Anz. 1989. 168:145–154.4. Tanriover N, Kawashima M, Rhoton AL, Ulm AJ, Mericle RA. Microsurgical anatomy of the early branches of the middle cerebral artery: morphometric analysis and classification with angiographic correlation. J Neurosurg. 2003. 98:1277–1290.
Article5. Sakamoto S, Ohba S, Shibukawa M, Kiura Y, Okazaki T, Arita K, et al. Characteristics of aneurysms of the internal carotid artery bifurcation. Acta Neurochir (Wien). 2006. 148:139–143.
Article6. Stehbens WE. Fox JL, editor. Etiology and pathogenesis of intracranial berry aneurysms. Intracranial aneurysms. 1938. New York: Springer-Verlag;358–395.
Article7. Ferguson GG. Physical factors in the initiation, growth, and rupture of human intracranial saccular aneurysms. J Neurosurg. 1972. 37:666–677.
Article8. Kayembe KN, Sasahara M, Hazama F. Cerebral aneurysms and variations of the circle of Willis. Stroke. 1984. 15:846–850.
Article9. Stehbens WE. Aneurysms and anatomical variation of cerebral arteries. Arch Pathol. 1963. 75:45–64.10. Ferguson GG. Turbulence in human intracranial saccular aneurysm. J Neurosurg. 1970. 33:485–497.11. Hashimoto T. Dynamic measurement of pressure and flow velocities in glass and silastic model berry aneurysms. Neurol Res. 1984. 6:22–28.
Article12. Stehbens WE. Flow in glass models of arterial bifurcations and berry aneurysms at low Reynolds numbers. Q J Exp Physiol Cogn Med Sci. 1975. 60:181–192.
Article13. Rhoton AL, Saeki N, Perlmutter D, Zeal A. Microsurgical anatomy of common aneurysm sites. Clin Neurosurg. 1979. 26:248–306.
Article14. Chrzanowski R. The assessment of the intracranial part of the internal carotid artery. Neuroradiology. 1971. 2:223–226.
Article15. Ferguson GG, Roach MR. Bergel DH, editor. Flow conditions at bifurcations as determined in glass models, with reference to the focal distribution of vascular lesions. Cardiovascular Fluid Dynamics. 1972. New York, NY: Academic Press;141–156.16. Liepsch DW. Flow in tubes and arteries: a comparison. Biorheology. 1986. 23:395–433.17. Nicholas WW, O'Rourke MF. McDonald's blood flow in arteries: Theoretical, experimental and clinical principles. 1990. 3rd ed. Philadelphia, PA: Lea and Febiger.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracranial Aneurysm Associated with Aplasia of the Internal Cartoid Artery
- Clinical Analysis of the Pattern of Anterior-Posterior Circulation in Patients with Posterior Communicating Artery Aneurysm
- Aneurysm at the Origin of the Uncal Artery Arising from the Internal Carotid Artery
- True Posterior Communicating Artery Aneurysm
- Transposition of Anterior Choroidal Artery and Posterior Communicating Artery Origin