J Korean Med Sci.
2012 Dec;27(12):1586-1590. 10.3346/jkms.2012.27.12.1586.
Reciprocal Deletion and Duplication of 17p11.2-11.2: Korean Patients with Smith-Magenis Syndrome and Potocki-Lupski Syndrome
- Affiliations
-
- 1Department of Pediatrics, Eulji General Hospital, Seoul, Korea.
- 2MG MED, Inc., Seoul, Korea.
- 3Department of Medical Genetics, Ajou University School of Medicine, Suwon, Korea. ybsohn@ajou.ac.kr
- 4Department of Physical Medicine and Rehabilitation, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2157987
- DOI: http://doi.org/10.3346/jkms.2012.27.12.1586
Abstract
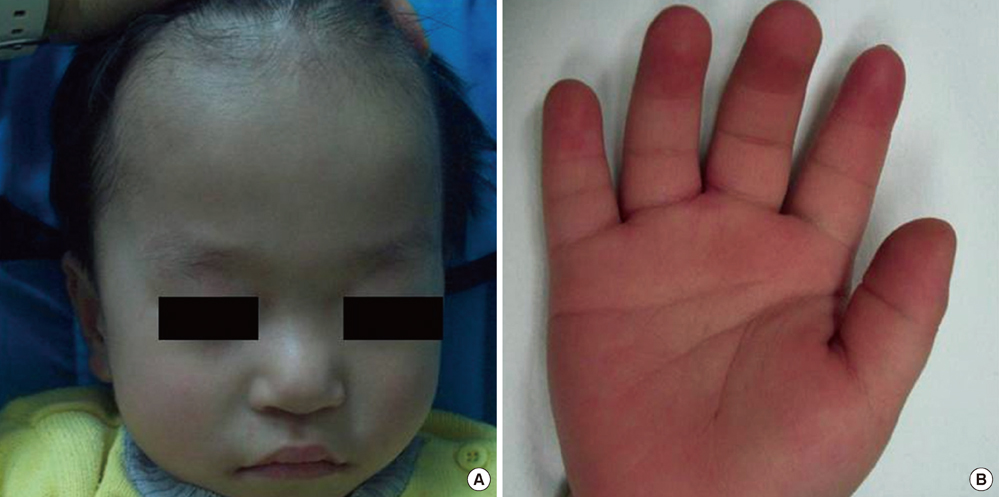
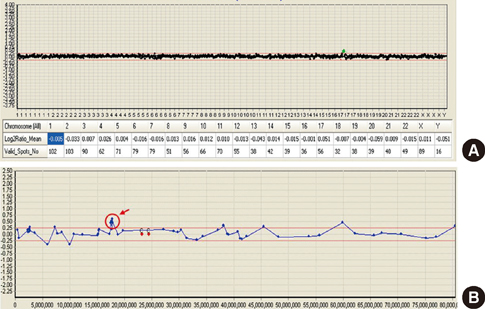
- Deletion and duplication of the -3.7-Mb region in 17p11.2 result in two reciprocal syndrome, Smith-Magenis syndrome and Potocki-Lupski syndrome. Smith-Magenis syndrome is a well-known developmental disorder. Potocki-Lupski syndrome has recently been recognized as a microduplication syndrome that is a reciprocal disease of Smith-Magenis syndrome. In this paper, we report on the clinical and cytogenetic features of two Korean patients with Smith-Magenis syndrome and Potocki-Lupski syndrome. Patient 1 (Smith-Magenis syndrome) was a 2.9-yr-old boy who showed mild dysmorphic features, aggressive behavioral problems, and developmental delay. Patient 2 (Potocki-Lupski syndrome), a 17-yr-old boy, had only intellectual disabilities and language developmental delay. We used array comparative genomic hybridization (array CGH) and found a 2.6 Mb-sized deletion and a reciprocal 2.1 Mb-sized duplication involving the 17p11.2. These regions overlapped in a 2.1 Mb size containing 11 common genes, including RAI1 and SREBF.
Keyword
MeSH Terms
-
Adolescent
Asian Continental Ancestry Group/*genetics
Child, Preschool
*Chromosomes, Human, Pair 17
Comparative Genomic Hybridization
Developmental Disabilities/etiology/genetics
Gene Deletion
Gene Duplication
Humans
Intellectual Disability/etiology/genetics
Karyotyping
Male
Smith-Magenis Syndrome/diagnosis/*genetics
Sterol Regulatory Element Binding Protein 1/genetics
Transcription Factors/genetics
Sterol Regulatory Element Binding Protein 1
Transcription Factors
Figure
Cited by 1 articles
-
Diagnosis of Smith-Magenis Syndrome in a Patient with Mental Retardation and Sleep Disturbance Confirmed by Multiplex Ligation-dependent Probe Amplification
Joowon Oh, Seungjae Lee, Kyung-A Lee, Jongha Yoo
Lab Med Online. 2018;8(2):71-74. doi: 10.3343/lmo.2018.8.2.71.
Reference
-
1. Morrow EM. Genomic copy number variation in disorders of cognitive development. J Am Acad Child Adolesc Psychiatry. 2010. 49:1091–1104.2. Shaw CJ, Bi W, Lupski JR. Genetic proof of unequal meiotic crossovers in reciprocal deletion and duplication of 17p11.2. Am J Hum Genet. 2002. 71:1072–1081.3. Bi W, Park SS, Shaw CJ, Withers MA, Patel PI, Lupski JR. Reciprocal crossovers and a positional preference for strand exchange in recombination events resulting in deletion or duplication of chromosome 17p11.2. Am J Hum Genet. 2003. 73:1302–1315.4. Gropman AL, Duncan WC, Smith AC. Neurologic and developmental features of the Smith-Magenis syndrome (del 17p11.2). Pediatr Neurol. 2006. 34:337–350.5. Potocki L, Bi W, Treadwell-Deering D, Carvalho CM, Eifert A, Friedman EM, Glaze D, Krull K, Lee JA, Lewis RA, et al. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am J Hum Genet. 2007. 80:633–649.6. Jones KL, Smith DW. Smith's recognizable patterns of human malformation. 2005. 6th edition. Philadelphia: Elsevier Saunders;210–212.7. Zody MC, Garber M, Adams DJ, Sharpe T, Harrow J, Lupski JR, Nicholson C, Searle SM, Wilming L, Young SK, et al. DNA sequence of human chromosome 17 and analysis of rearrangement in the human lineage. Nature. 2006. 440:1045–1049.8. Shchelochkov OA, Cheung SW, Lupski JR. Genomic and clinical characteristics of microduplications in chromosome 17. Am J Med Genet A. 2010. 152A:1101–1110.9. Zhang F, Potocki L, Sampson JB, Liu P, Sanchez-Valle A, Robbins-Furman P, Navarro AD, Wheeler PG, Spence JE, Brasington CK, et al. Identification of uncommon recurrent Potocki-Lupski syndrome-associated duplications and the distribution of rearrangement types and mechanisms in PTLS. Am J Hum Genet. 2010. 86:462–470.10. Walz K, Spencer C, Kaasik K, Lee CC, Lupski JR, Paylor R. Behavioral characterization of mouse models for Smith-Magenis syndrome and dup(17)(p11.2p11.2). Hum Mol Genet. 2004. 13:367–378.11. Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith AC, Elsea SH. Gender, genotype, and phenotype differences in Smith-Magenis syndrome: a meta-analysis of 105 cases. Clin Genet. 2007. 71:540–550.12. Walz K, Paylor R, Yan J, Bi W, Lupski JR. Rai1 duplication causes physical and behavioral phenotypes in a mouse model of dup(17)(p11.2p11.2). J Clin Invest. 2006. 116:3035–3041.13. Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, Reymond A, Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum Mol Genet. 2008. 17:2486–2495.14. Madduri N, Peters SU, Voigt RG, Llorente AM, Lupski JR, Potocki L. Cognitive and adaptive behavior profiles in Smith-Magenis syndrome. J Dev Behav Pediatr. 2006. 27:188–192.15. Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005. 33:D514–D517.16. Jung SK, Park KH, Shin HK, Eun SH, Eun BL, Yoo KH, Hong YS, Lee JW, Bae SY. Two cases of Smith-Magenis syndrome. Korean J Pediatr. 2009. 52:701–704.17. Suk JH, Lee SG, Bae JC, Lee HJ, Kim SH. Two Cases of Smith-Magenis Syndrome with Tetralogy of Fallot Confirmed by FISH. Korean J Lab Med. 2005. 25:361–364.18. Cho EH, Park BYN, Cho JH, Kang YS. Comparing two diagnostic laboratory tests for several microdeletions causing mental retardation syndromes: multiplex ligation-fependent amplification vs fluorescent in situ hybridization. Korean J Lab Med. 2009. 29:71–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two cases of Smith-Magenis syndrome
- Two Cases of Smith-Magenis Syndrome with Tetralogy of Fallot Confirmed by FISH
- A Case of Smith-Magenis Syndrome with Multiple Organ Malformations
- Identification of Potocki–Lupski syndrome in patients with developmental delay and growth failure
- Diagnosis of Smith-Magenis Syndrome in a Patient with Mental Retardation and Sleep Disturbance Confirmed by Multiplex Ligation-dependent Probe Amplification