J Korean Med Sci.
2012 Apr;27(4):446-449. 10.3346/jkms.2012.27.4.446.
Immunoglobulin A Nephropathy Associated with Plasmodium falciparum Malaria
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. hansh@yuhs.ac
- 2Department of Internal Medicine, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- 3Department of Pathology, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- KMID: 2157908
- DOI: http://doi.org/10.3346/jkms.2012.27.4.446
Abstract
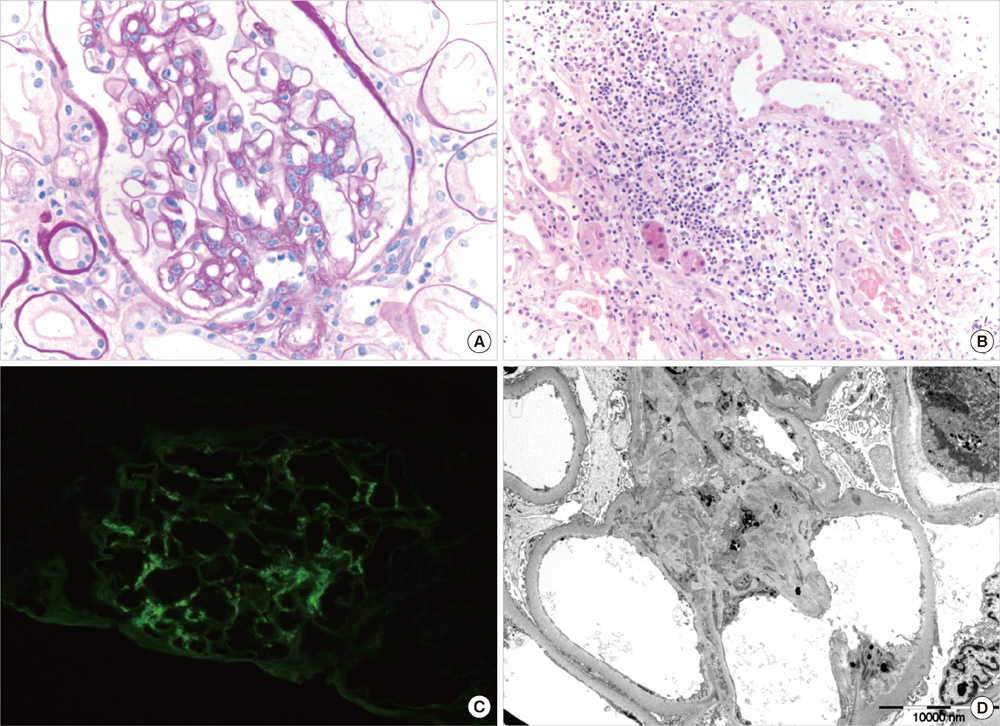
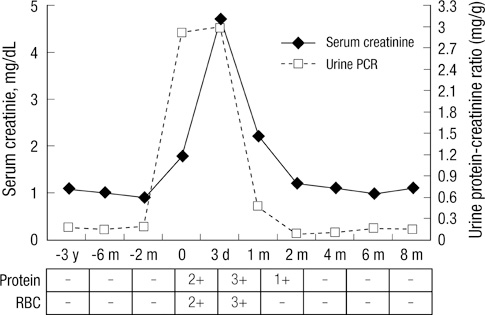
- Glomerulonephritis occurs as a rare form of renal manifestation in Plasmodium falciparum malaria. Herein, we report a case of falciparum malaria-associated IgA nephropathy for the first time. A 49-yr old male who had been to East Africa was diagnosed with Plasmodium falciparum malaria. Microhematuria and proteinuria along with acute kidney injury developed during the course of the disease. Kidney biopsy showed mesangial proliferation and IgA deposits with tubulointerstitial inflammation. Laboratory tests after recovery from malaria showed disappearance of urinary abnormalities and normalization of kidney function. Our findings suggest that malaria infection might be associated with IgA nephropathy.
MeSH Terms
-
Acute Kidney Injury/etiology/pathology
Antimalarials/therapeutic use
Creatinine/blood
Glomerulonephritis, IGA/*diagnosis/*etiology
Hematuria/etiology
Humans
Immunoglobulin A/*metabolism
Malaria/*complications/drug therapy/*pathology
Male
Middle Aged
Plasmodium falciparum/*isolation & purification
Proteinuria/etiology
Quinine/therapeutic use
Figure
Reference
-
1. World Health Organization. World malaria report 2009. 2009. Geneva: World Health Organization.2. Barsoum RS. Malarial nephropathies. Nephrol Dial Transplant. 1998. 13:1588–1597.3. Barsoum RS, Sitprija V. Schrier RW, editor. Tropical nephrology. Diseases of the kidney and urinary tract. 2001. . Philadelphia: Lippincott Williams & Wilkins;2013–2020.4. Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002. 347:738–748.5. Sunaga H, Oh M, Takahashi N, Fujieda S. Infection of Haemophilus parainfluenzae in tonsils is associated with IgA nephropathy. Acta Otolaryngol Suppl. 2004. 15–19.6. Satoskar AA, Nadasdy G, Plaza JA, Sedmak D, Shidham G, Hebert L, Nadasdy T. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol. 2006. 1:1179–1186.7. Suzuki K, Hirano K, Onodera N, Takahashi T, Tanaka H. Acute IgA nephropathy associated with mycoplasma pneumoniae infection. Pediatr Int. 2005. 47:583–585.8. Wang NS, Wu ZL, Zhang YE, Guo MY, Liao LT. Role of hepatitis B virus infection in pathogenesis of IgA nephropathy. World J Gastroenterol. 2003. 9:2004–2008.9. Upadhaya BK, Sharma A, Khaira A, Dinda AK, Agarwal SK, Tiwari SC. Transient IgA nephropathy with acute kidney injury in a patient with dengue fever. Saudi J Kidney Dis Transpl. 2010. 21:521–525.10. George CR, Parbtani A, Cameron JS. Mouse malaria nephropathy. J Pathol. 1976. 120:235–249.11. Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004. 24:197–217.12. Coppo R, Amore A. Aberrant glycosylation in IgA nephropathy (IgAN). Kidney Int. 2004. 65:1544–1547.13. Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999. 104:73–81.14. Hamadeh RM, Galili U, Zhou P, Griffiss JM. Anti-alpha-galactosyl immunoglobulin A (IgA), IgG, and IgM in human secretions. Clin Diagn Lab Immunol. 1995. 2:125–131.15. Davin JC, Malaise M, Foidart J, Mahieu P. Anti-alpha-galactosyl antibodies and immune complexes in children with Henoch-Schönlein purpura or IgA nephropathy. Kidney Int. 1987. 31:1132–1139.16. Ravindran B, Satapathy AK, Das MK. Naturally-occurring anti-alpha-galactosyl antibodies in human Plasmodium falciparum infections: a possible role for autoantibodies in malaria. Immunol Lett. 1988. 19:137–141.17. Ramasamy R, Reese RT. Terminal galactose residues and the antigenicity of Plasmodium falciparum glycoproteins. Mol Biochem Parasitol. 1986. 19:91–101.18. Jakobsen PH, Theander TG, Jensen JB, Mølbak K, Jepsen S. Soluble Plasmodium falciparum antigens contain carbohydrate moieties important for immune reactivity. J Clin Microbiol. 1987. 25:2075–2079.19. Han SH, Kang EW, Kie JH, Yoo TH, Choi KH, Han DS, Kang SW. Spontaneous remission of IgA nephropathy associated with resolution of hepatitis A. Am J Kidney Dis. 2010. 56:1163–1167.20. Nasr SH, D'Agati VD. IgA-dominant postinfectious glomerulonephritis: a new twist on an old disease. Nephron Clin Pract. 2011. 119:c18–c25.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple Cerebral Infarcts Following Acute Plasmodium vivax Infection
- Intraleukocytic hemozoin pigments in complicated Plasmodium falciparum cerebral malaria
- Treatment outcomes of imported Plasmodium falciparum malaria with intravenous artesunate
- Blackwater Fever Followed by Severe Falciparum Malaria in a Child
- Primaquine Administration after Falciparum Malaria Treatment in Malaria Hypoendemic Areas with High Incidence of Falciparum and Vivax Mixed Infection: Pros and Cons