J Korean Med Sci.
2005 Dec;20(6):961-965. 10.3346/jkms.2005.20.6.961.
Emergence and Wide Dissemination of CTX-M-type ESBLs, and CMY-2- and DHA-1-type AmpC beta-Lactamases in Korean Respiratory Isolates of Klebsiella pneumoniae
- Affiliations
-
- 1Department of Laboratory Medicine, Research Institute of Bacterial Resistance, and Brain Korea 21 Medical Sciences, Yonsei University College of Medicine, Seoul, Korea. leekcp@yumc.yonsei.ac.kr
- 2Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2157745
- DOI: http://doi.org/10.3346/jkms.2005.20.6.961
Abstract
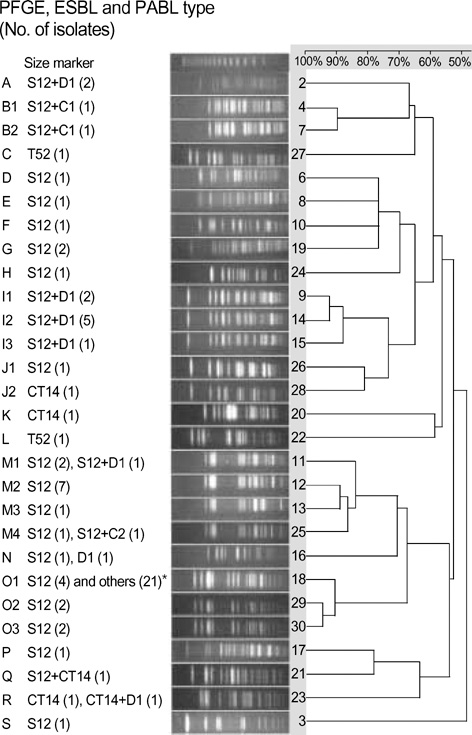
- Respiratory isolates of Klebsiella pneumoniae in Korea during 2002-2003 were studied to determine the prevalence and types of extended-spectrum beta-lactamases (ESBLs) and plasmid-mediated AmpC beta-lactamases (PABLs). ESBL-production was tested by double-disk synergy, and genotypes of beta-lactamases were determined by PCR and sequencing. ESBLs were detected in 28.4% of 373 isolates, and the most prevalent types were SHV-12 (63 isolates) and CTX-M-14 (9 isolates). Forty of 75 ESBL-producers (53.5%) also had PABLs: 21 isolates with CMY-2-like, 17 with DHA-1-like. Pulsed-field gel electrophoresis showed 19 types and 25 of 74 isolates had an identical pattern, indicating nosocomial spread. Dissemination of ESBL- and PABL-producing K. pneumoniae strains in Korea is a particular concern, as it limits the choice of antimicrobial agents for treatment of infections.
Keyword
MeSH Terms
-
Anti-Bacterial Agents/therapeutic use
Bacterial Proteins/biosynthesis/classification/genetics
Base Sequence
Cross Infection/microbiology
DNA, Bacterial/genetics
Drug Resistance, Bacterial
Electrophoresis, Gel, Pulsed-Field
Genes, Bacterial
Humans
Klebsiella Infections/drug therapy/*microbiology
Klebsiella pneumoniae/classification/*enzymology/genetics/*isolation and purification
Korea
Research Support, Non-U.S. Gov't
Respiratory Tract Infections/drug therapy/*microbiology
beta-Lactamases/biosynthesis/*classification/genetics
Figure
Reference
-
1. Bradford PA. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001. 14:933–951.
Article2. Pai H. The characteristics of extended-spectrum β-lactamases in Korean isolates of Enterobacteriaceae. Yonsei Med J. 1998. 39:514–519.3. Pai H, Choi EH, Lee HJ, Hong JY, Jacoby GA. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J Clin Microbiol. 2001. 39:3747–3749.
Article4. Bauernfeind A, Chong Y, Schweighart S. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection. 1989. 17:316–321.
Article5. Bauernfeind A, Chong Y, Lee K. Plasmid-encoded AmpC β-lactamases: How far have we gone 10 years after the discovery? Yonsei Med J. 1998. 39:520–525.
Article6. Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type β-lactamases. Antimicrob Agents Chemother. 2002. 46:1–11.
Article7. Yan JJ, Ko WC, Wu HM, Tsai SH, Chuang CL, Wu JJ. Complexity of Klebsiella pneumoniae isolates resistant to both cephamycins and extended-spectrum cephalosporins at a Teaching Hospital in Taiwan. J Clin Microbiol. 2004. 42:5337–5340.
Article8. Farmer JJ III. Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Enterobacteriaceae: introduction and identification. Manual of Clinical Microbiology. 1999. 7th ed. Washington, DC: ASM.9. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; Twelfth informational supplement. 2002. Wayne, PA: NCCLS;M100-S12.10. Hong SG, Kim S, Jeong SH, Chang CL, Cho SR, Ahn JY, Shin JH, Lee HS, Song WK, Uh Y, Yum JH, Yong D, Lee K, Chong Y. Prevalence and diversity of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates in Korea. Korean J Clin Microbiol. 2003. 6:149–155.11. Perez-Perez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002. 40:2153–2162.
Article12. Lee SH, Jeong SH, Park YM. Characterization of blaCMY-10 a novel, plasmid-encoded AmpC-type β-lactamase gene in a clinical isolate of Enterobacter aerogenes. J Appl Microbiol. 2003. 95:744–752.13. Lee SH, Kim JY, Lee GS, Cheon SH, An YJ, Jeong SH, Lee KJ. Characterization of blaCMY-11, an AmpC-type plasmid-mediated β-lactamase gene in a Korean clinical isolate of Escherichia coli. J Antimicrob Chemother. 2002. 49:269–273.14. Lee K, Jang SJ, Lee HJ, Ryoo N, Kim M, Hong SG, Chong Y. Increasing prevalence of vancomycin-resistant Enterococcus faecium, expanded-spectrum cephalosporin-resistant Klebsiella pneumoniae, and imipenem-resistant Pseudomonas aeruginosa in Korea: KONSAR study in 2001. J Korean Med Sci. 2004. 19:8–14.15. Bauernfeind A, Stemplinger I, Jungwirth R, Giamarellou H. Characterization of the plasmid β-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob Agents Chemother. 1996. 40:221–224.16. Poirel L, Heritier C, Spicq C, Nordmann P. In vivo acquisition of high-level resistance to imipenem in Escherichia coli. J Clin Microbiol. 2004. 42:3831–3833.
Article17. Bradford PA, Urban C, Mariano N, Projan SJ, Rahal JJ, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997. 41:563–569.18. Gaillot O, Clement C, Simonet M, Philippon A. Novel transferable β-lactam resistance with cephalosporinase characteristics in Salmonella enteritidis. J Antimicrob Chemother. 1997. 39:85–87.19. Kim JY, Park YJ, Lee SO, Song W, Jeong SH, Yoo YA, Lee KY. Case report: Bacteremia due to Salmonella enterica serotype Montevideo producing plasmid-mediated AmpC beta-lactamase (DHA-1). Ann Clin Lab Sci. 2004. 34:214–217.20. Pai H, Kang CI, Byeon JH, Lee KD, Park WB, Kim HB, Kim EC, Oh MD, Choe KW. Epidemiology and clinical features of bloodstream infections caused by AmpC-type-β-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004. 48:3720–3728.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- AmpC-type beta-lactamases in Clinical Isolated of Cefoxitin-resistant E. coli and K. pneumoniae
- An Increase in the Clinical Isolation of Acquired AmpC beta-Lactamase-Producing Klebsiella pneumoniae in Korea from 2007 to 2010
- Characterization of Extended-spectrum Beta-lactamases in Klebsiella pneumoniae Isolated in Korea
- Epidemiology of Plasmid-Mediated AmpC beta-Lactamase Produced by Clinical Isolates of Klebsiella pneumoniae from Four University Hospitals in Korea
- The characteristics of extended-spectrum beta-lactamases in Korean isolates of Enterobacteriaceae