Ann Dermatol.
2015 Dec;27(6):721-726. 10.5021/ad.2015.27.6.721.
A Study about the Cause and Clinicopathologic Findings of Injection-Induced Dermatitis
- Affiliations
-
- 1Department of Dermatology, Kyung Hee University College of Medicine, Seoul, Korea. bellotte@hanmail.net
- KMID: 2157449
- DOI: http://doi.org/10.5021/ad.2015.27.6.721
Abstract
- BACKGROUND
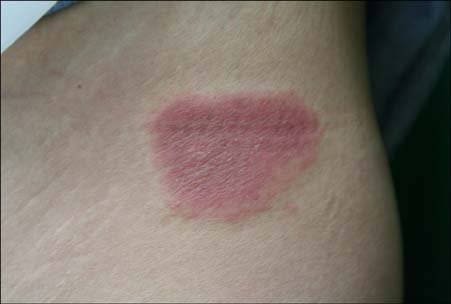
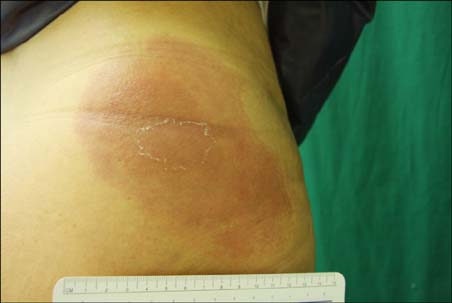
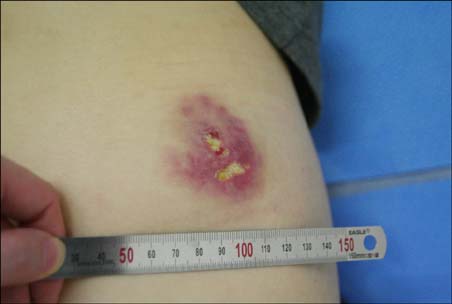
Cases of dermatitis induced by the injection of certain drugs have been reported.
OBJECTIVE
The aim of this study was to assess the cause and clinicopathologic findings of injection-induced dermatitis, and to reveal whether the reaction has any relation to the patient's age, injection site, drug concentration, and time interval from the injection to the occurrence of the skin lesion.
METHODS
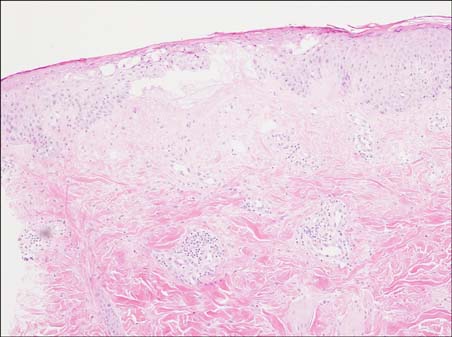
In this study, we enrolled 10 patients who developed erythematous skin lesions after the injection of causative drugs. The lesions were compared to each other according to the injection site, time interval from the injection to the occurrence of the skin lesion, and clinical characteristics. We performed intradermal and patch tests in each patient with different concentrations of causative drugs.
RESULTS
The most common causative drugs were diclofenac and vitamin K1. The eczematous type was the most frequent clinical type. The intradermal test showed more positive results than the patch test. The patch tests with diclofenac (as is, 2.5%, 5%, and 10%) and vitamin K1 (10%) were all negative in 10 patients. Furthermore, intradermal tests with diclofenac (as is) and vitamin K1 (0.1%, 1%, and 10%) were performed in 8 patients. Six patients had a positive reaction, consisting of erythema, induration, and vesiculation, after 1 and 2 days.
CONCLUSION
Our results showed that the most common causative agents were diclofenac and vitamin K1. Moreover, it seems that that intradermal test is more useful than the patch test in the diagnosis of injection-induced dermatitis.
Keyword
MeSH Terms
Figure
Reference
-
1. Seo SH, Jang HS, Jang BS, Kim MB, Oh CK, Kwon KS. A case of Vitamin K1 dermatitis. Korean J Dermatol. 2006; 44:341–345.2. Bruynzeel I, Hebeda CL, Folkers E, Bruynzeel DP. Cutaneous hypersensitivity reactions to vitamin K: 2 case reports and a review of the literature. Contact Dermatitis. 1995; 32:78–82.
Article3. Chang HS, Moon HS, Lee JH, Park K, Son SJ. A case of Nicolau syndrome treated with non-steroidal anti-inflammatory drug injection therapy. Korean J Dermatol. 2009; 47:459–462.4. Kim KK. Nicolau syndrome in patient following diclofenac administration: a case report. Ann Dermatol. 2011; 23:501–503.
Article5. Todd PA, Sorkin EM. Diclofenac sodium. A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1988; 35:244–285.6. Joyce JP, Hood AF, Weiss MM. Persistent cutaneous reaction to intramuscular vitamin K injection. Arch Dermatol. 1988; 124:27–28.
Article7. Wong DA, Freeman S. Cutaneous allergic reaction to intramuscular vitamin K1. Australas J Dermatol. 1999; 40:147–152.8. Finkelstein H, Champion MC, Adam JE. Cutaneous hypersensitivity to vitamin K1 injection. J Am Acad Dermatol. 1987; 16:540–545.
Article9. Dinis A, Brandão M, Faria A. Occupational contact dermatitis from vitamin K3 sodium bisulphite. Contact Dermatitis. 1988; 18:170–171.
Article10. Barnes HM, Sarkany I. Adverse skin reaction from vitamin K1. Br J Dermatol. 1976; 95:653–656.11. Lemlich G, Green M, Phelps R, Lebwohl M, Don P, Gordon M. Cutaneous reactions to vitamin K1 injections. J Am Acad Dermatol. 1993; 28:345–347.
Article12. Bullen AW, Miller JP, Cunliffe WJ, Losowsky MS. Skin reactions caused by vitamin K in patients with liver disease. Br J Dermatol. 1978; 98:561–565.
Article13. Robison JW, Odom RB. Delayed cutaneous reaction to phytonadione. Arch Dermatol. 1978; 114:1790–1792.
Article14. Heydenreich G. A further case of adverse skin reaction from vitamin K1. Br J Dermatol. 1977; 97:697.
Article15. Sanders MN, Winkelmann RK. Cutaneous reactions to vitamin K. J Am Acad Dermatol. 1988; 19:699–704.
Article16. Rietschel RL, Fowler JF. Vehicles and preservatives including formaldehyde, cosmetics, and personal-care products. In : Rietschel RL, Fowler JF, editors. Fisher's Contact Dermatitis. 4th ed. Baltimore: Williams and Wilkins;1995. p. 257–329.17. Pigatto PD, Bigardi A, Fumagalli M, Altomare GF, Riboldi A. Allergic dermatitis from parenteral vitamin K. Contact Dermatitis. 1990; 22:307–308.
Article18. Draper G, McNinch A. Vitamin K for neonates: the controversy. BMJ. 1994; 308:867–868.
Article19. Pang BK, Munro V, Kossard S. Pseudoscleroderma secondary to phytomenadione (vitamin K1) injections: Texier's disease. Australas J Dermatol. 1996; 37:44–47.
Article20. Guidetti MS, Vincenzi C, Papi M, Tosti A. Sclerodermatous skin reaction after vitamin K1 injections. Contact Dermatitis. 1994; 31:45–46.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Localized Erythematous Dermatitis on the Site of a Docetaxel Injection
- Role of IL-10 in the Trimellitic Anhydride-induced Contact Dermatitis
- Exfoliative Dermatitis Induced by Minocycline
- The Clinicopathologic Study of Foreign Body Granuloma Induced by Injection of Filler
- A Study on the Cell - Mediated Immunity of Patients with Apopic Dermatitis