Immune Netw.
2012 Dec;12(6):291-295. 10.4110/in.2012.12.6.291.
The Role of the Ethylacetate Fraction from Hydnocarpi Semen in Acute Inflammation In Vitro Model
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul 139-742, Korea. kangtj@syu.ac.kr
- 2Uimyung Research Institute for Neuroscience, Sahmyook University, Seoul 139-742, Korea.
- 3Institute of Chronic Disease, Sahmyook University, Seoul 139-742, Korea.
- KMID: 2150762
- DOI: http://doi.org/10.4110/in.2012.12.6.291
Abstract
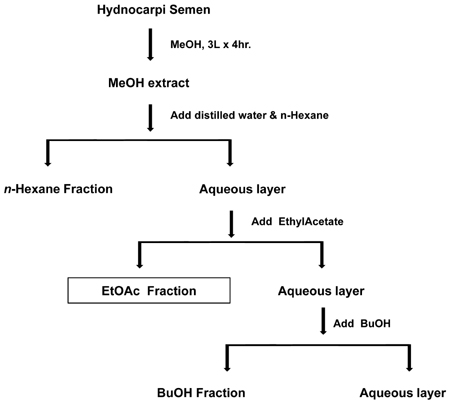
- We previously reported that Hydnocarpi Semen (HS) has a wound healing effect on diabetic foot ulcer lesion in mice. In this study, ethylacetate (EtOAc) fraction from HS extract were evaluated for their wound healing activity by using in vitro acute inflammation model. GC and GC/MS analysis shows that the main constituents in EtOAc fraction are chaulmoogric acid, hydnocarpic acid, and gorlic acid. EtOAc fraction activated macrophages to increase the production of TNF-alpha. The fraction also increased the production of TGF-beta and VEGF, which induced fibroblast activation and angiogenesis. These results suggest that the mechanism that the fraction helps to enhance healing of skin wound is possibly associated with the production of TNF-alpha, as well as secretion of VEGF, TGF-beta and HS may have a new bioactive material for the treatment of skin wound.
MeSH Terms
-
Animals
Cytokines
Diabetic Foot
Fatty Acids
Fibroblasts
Inflammation
Macrophages
Mice
Semen
Skin
Transforming Growth Factor beta
Tumor Necrosis Factor-alpha
Ulcer
Vascular Endothelial Growth Factor A
Wound Healing
Cytokines
Fatty Acids
Transforming Growth Factor beta
Tumor Necrosis Factor-alpha
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999. 341:738–746.
Article2. Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006. 11:128–150.3. Levy L. The activity of chaulmoogra acids against Mycobacterium leprae. Am Rev Respir Dis. 1975. 111:703–705.4. Oommen ST, Rao M, Raju CV. Effect of oil of hydnocarpus on wound healing. Int J Lepr Other Mycobact Dis. 1999. 67:154–158.5. Oommen ST. The effect of oil of hydnocarpus on excision wounds. Int J Lepr Other Mycobact Dis. 2000. 68:69–70.6. Lee GS, Choi JY, Choi YJ, Yim DS, Kang TJ, Cheong JH. The wound healing effect of Hydnocarpi Semen extract on ulcer in diabetic mice. Biomol Ther. 2010. 18:329–335.
Article7. Lee GS, Yim D, Cheong JH, Kang TJ. The n-Hexane, ethylacetate, and butanol fractions from Hydnocarpi Semen enhanced wound healing in a mice ulcer model. Immunopharmacol Immunotoxicol. 2012. 34:968–974.
Article8. Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, Li Z, Croitoru A, Thung S, Vlassara H. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003. 52:2805–2813.
Article9. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007. 6:273–286.
Article10. Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991. 88:4220–4224.
Article11. Vanderslice P, Munsch CL, Rachal E, Erichsen D, Sughrue KM, Truong AN, Wygant JN, McIntyre BW, Sughrue KM, Eskin SG, Tilton RG, Polverini PJ. Angiogenesis induced by tumor necrosis factor-agr; is mediated by alpha4 integrins. Angiogenesis. 1998. 2:265–275.12. Raghow R. The role of extracellular matrix in post inflammatory wound healing and fibrosis. FASEB J. 1994. 8:823–831.
Article13. Michaels J 5th, Dobryansky M, Galiano RD, Bhatt KA, Ashinoff R, Ceradini DJ, Gurtner GC. Topical vascular endothelial growth factor reverses delayed wound healing secondary to angiogenesis inhibitor administration. Wound Repair Regen. 2005. 13:506–512.
Article14. Saaristo A, Tammela T, Farkkilã A, Kärkkäinen M, Suominen E, Yla-Herttuala S, Alitalo K. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol. 2006. 169:1080–1087.
Article15. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001. 114:853–865.
Article16. Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006. 117:7 Suppl. 12S–34S.
Article17. Amendt C, Mann A, Schirmacher P, Blessing M. Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J Cell Sci. 2002. 115:2189–2198.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on anticancer effects of extracts caesalpinia sappan on oral carcinoma and osteosarcoma cells
- The Effects of Pulsatilla Koreana for Anti-Inflammatory and Cellular Activity of Periodontal Tissue
- Anticancer effects of caesalpinia sappan extracts on oral carcinoma and osteosarcoma cells
- Changes of Prostaglandins in the Semen from Patients with Prostatitis
- Evaluation of routine semen analysis by means of Hamilton-Thorn 2000 motility analyzer