Immune Netw.
2010 Dec;10(6):188-197. 10.4110/in.2010.10.6.188.
Dendritic Cell Activation by Glucan Isolated from Umbilicaria Esculenta
- Affiliations
-
- 1College of Pharmacy and Medical Research Center (CICT), Chungbuk National University, Cheongju 361-763, Korea. shan@chungbuk.ac.kr
- 2Macrocare, Ochang 363-883, Korea.
- 3Korea Research Institute of Bioscience and Biotechnology, Ochang 363-883, Korea.
- 4Institute of Natural Sciences, Yong-In University, Yongin 449-714, Korea.
- KMID: 2150674
- DOI: http://doi.org/10.4110/in.2010.10.6.188
Abstract
- BACKGROUND
Lichen-derived glucans have been known to stimulate the functions of immune cells. However, immunostimulatory activity of glucan obtained from edible lichen, Umbilicaria esculenta, has not been reported. Thus we evaluated the phenotype and functional maturation of dendritic cells (DCs) following treatment of extracted glucan (PUE).
METHODS
The phenotypic and functional maturation of PUE-treated DCs was assessed by flow cytometric analysis and cytokine production, respectively. PUE-treated DCs was also used for mixed leukocyte reaction to evaluate T cell-priming capacity. Finally we detected the activation of MAPK and NF-kappaB by immunoblot.
RESULTS
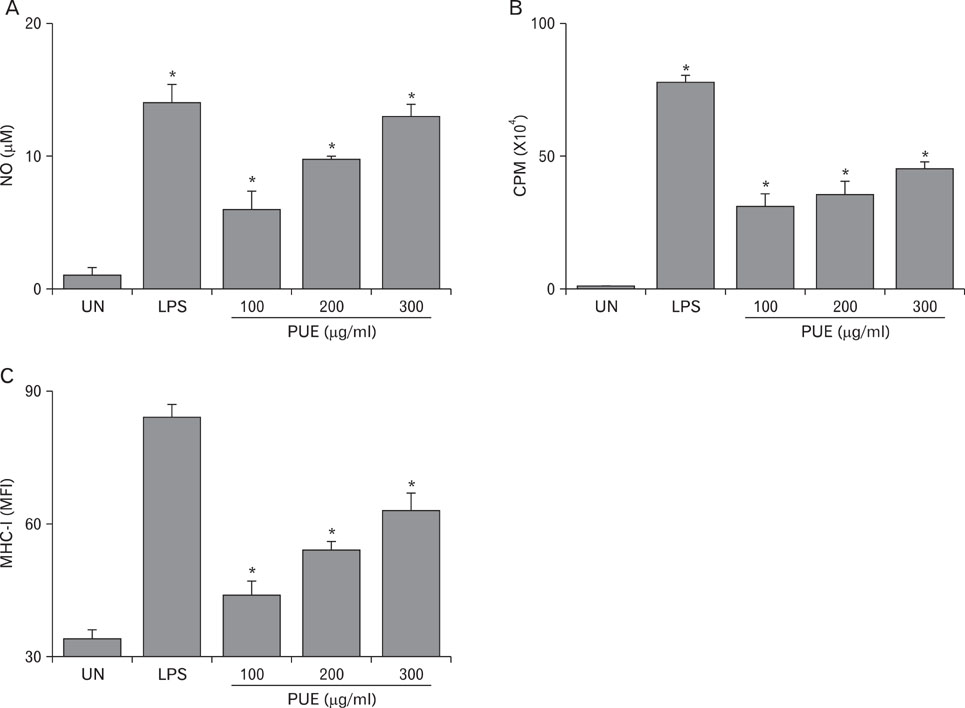
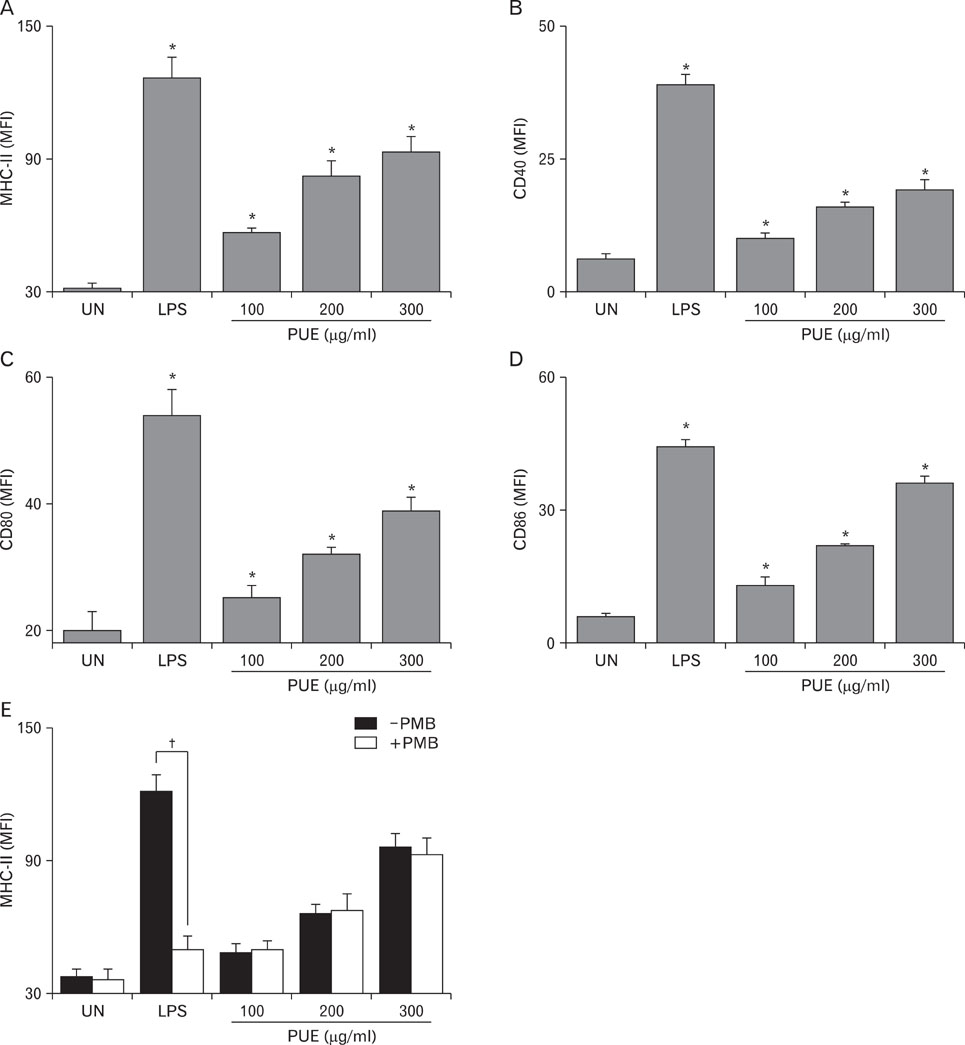
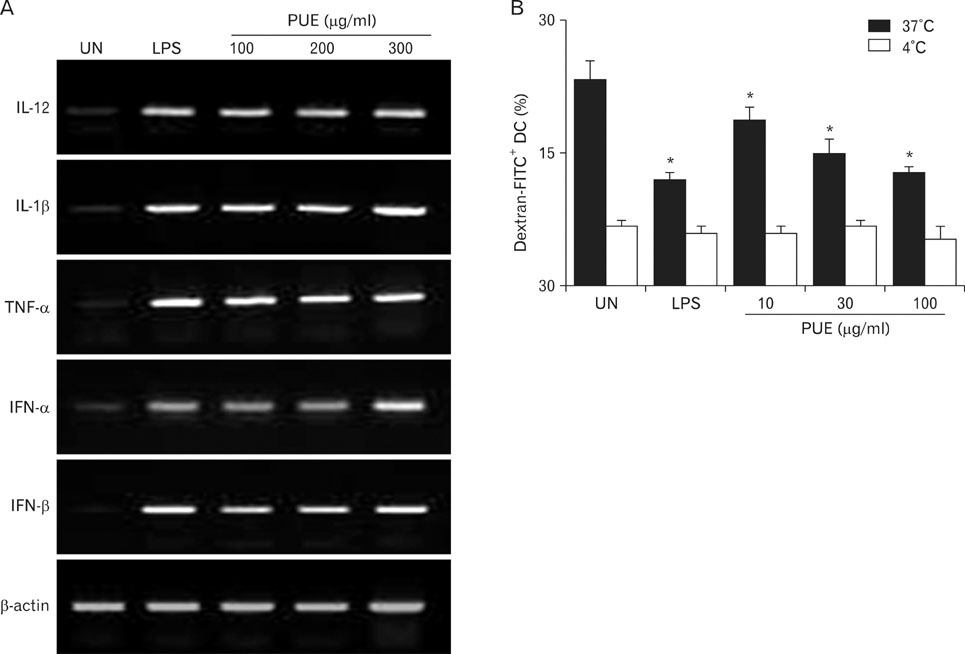
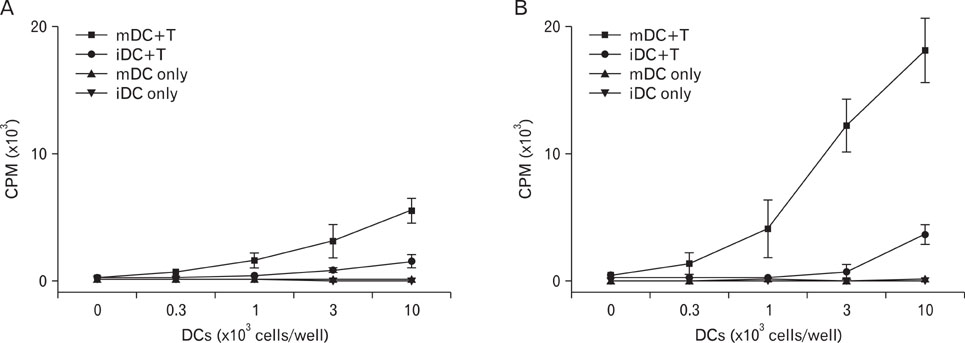
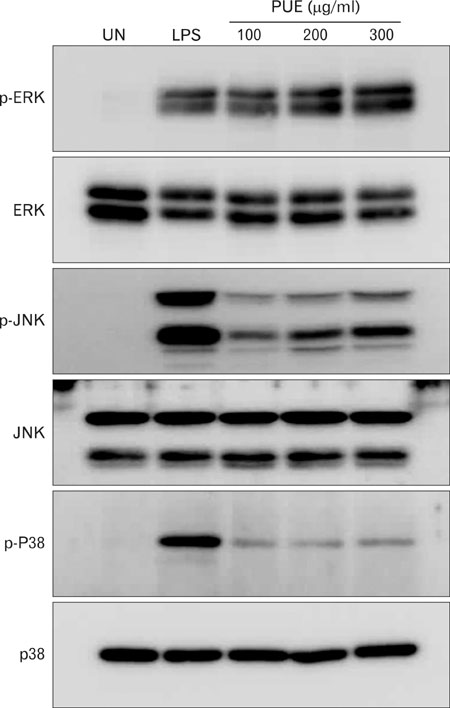
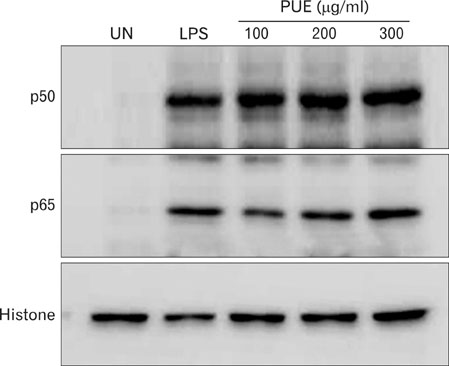
Phenotypic maturation of DCs was shown by the elevated expressions of CD40, CD80, CD86, and MHC class I/II molecules. Functional activation of DCs was proved by increased cytokine production of IL-12, IL-1beta, TNF-alpha, and IFN-alpha/beta, decreased endocytosis, and enhanced proliferation of allogenic T cells. Polymyxin B, specific inhibitor of lipopolysaccharide (LPS), did not affect PUE activity, which suggested that PUE was free of LPS contamination. As a mechanism of action, PUE increased phosphorylation of ERK, JNK, and p38 MAPKs, and enhanced nuclear translocation of NF-kappaB p50/p65 in DCs.
CONCLUSION
These results indicate that PUE induced DC maturation via MAPK and NF-kappaB signaling pathways.
Keyword
MeSH Terms
-
Dendritic Cells
Endocytosis
Glucans
Interleukin-12
Lichens
Lymphocyte Culture Test, Mixed
NF-kappa B
p38 Mitogen-Activated Protein Kinases
Phenotype
Phosphorylation
Polymyxin B
T-Lymphocytes
Tumor Necrosis Factor-alpha
Glucans
Interleukin-12
NF-kappa B
Polymyxin B
Tumor Necrosis Factor-alpha
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006. 6:317–333.
Article2. Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 2000. 13:523–533.
Article3. Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002. 60:258–274.
Article4. Nakano H, Namatame K, Nemoto H, Motohashi H, Nishiyama K, Kumada K. Kanagawa Lentinan Research Group. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Hepatogastroenterology. 1999. 46:2662–2668.5. Okamura K, Suzuki M, Chihara T, Fujiwara A, Fukuda T, Goto S, Ichinohe K, Jimi S, Kasamatsu T, Kawai N, et al. Clinical evaluation of schizophyllan combined with irradiation in patients with cervical cancer. A randomized controlled study. Cancer. 1986. 58:865–872.
Article6. Pang ZJ, Zhou M, Chen Y, Wan J. A protein-bound polysaccharide synergistic with lipopolysaccharide induces nitric oxide release and antioxidant enzyme activities in mouse peritoneal macrophages. Am J Chin Med. 1998. 26:133–141.
Article7. Okazaki M, Adachi Y, Ohno N, Yadomae T. Structure-activity relationship of (1-->3)-beta-D-glucans in the induction of cytokine production from macrophages, in vitro. Biol Pharm Bull. 1995. 18:1320–1327.
Article8. Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med. 1999. 221:281–293.
Article9. Ingolfsdottir K, Jurcic K, Fischer B, Wagner H. Immunologically active polysaccharide from Cetraria islandica. Planta Med. 1994. 60:527–531.
Article10. Freysdottir J, Omarsdottir S, Ingólfsdóttir K, Vikingsson A, Olafsdottir ES. In vitro and in vivo immunomodulating effects of traditionally prepared extract and purified compounds from Cetraria islandica. Int Immunopharmacol. 2008. 8:423–430.
Article11. Olafsdottir ES, Ingolfsdottir K, Barsett H, Paulsen BS, Jurcic K, Wagner H. Immunologically active (1-->3)-(1-->4)-alpha-D-glucan from Cetraria islandica. Phytomedicine. 1999. 6:33–39.
Article12. Omarsdottir S, Freysdottir J, Olafsdottir ES. Immunomodulating polysaccharides from the lichen Thamnolia vermicularis var. subuliformis. Phytomedicine. 2007. 14:179–184.
Article13. Olafsdottir ES, Omarsdottir S, Paulsen BS, Wagner H. Immunologically active O6-branched (1-->3)-beta-glucan from the lichen Thamnolia vermicularis var. subuliformis. Phytomedicine. 2003. 10:318–324.
Article14. Kim MS, Lee KA. Antithrombotic activity of methanolic extract of Umbilicaria esculenta. J Ethnopharmacol. 2006. 105:342–345.
Article15. Kim MS, Cho HB. Melanogenesis inhibitory effects of methanolic extracts of Umbilicaria esculenta and Usnea longissima. J Microbiol. 2007. 45:578–582.16. Lee KA, Kim MS. Glucosidase inhibitor from Umbilicaria esculenta. Can J Microbiol. 2000. 46:1077–1081.17. Kim HS, Kim JY, Ryu HS, Park HG, Kim YO, Kang JS, Kim HM, Hong JT, Kim Y, Han SB. Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. Int Immunopharmacol. 2010. 10:1284–1294.
Article18. Kim JY, Kang JS, Kim HM, Ryu HS, Kim HS, Lee HK, Kim YJ, Hong JT, Kim Y, Han SB. Inhibition of bone marrow-derived dendritic cell maturation by glabridin. Int Immunopharmacol. 2010. 10:1185–1193.
Article19. Kim JY, Yoon YD, Ahn JM, Kang JS, Park SK, Lee K, Song KB, Kim HM, Han SB. Angelan isolated from Angelica gigas Nakai induces dendritic cell maturation through toll-like receptor 4. Int Immunopharmacol. 2007. 7:78–87.
Article20. Kim HS, Kim JY, Kang JS, Kim HM, Kim YO, Hong IP, Lee MK, Hong JT, Kim Y, Han SB. Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food Chem Toxicol. 2010. 48:1926–1933.
Article21. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000. 18:767–811.
Article22. Bennaceur K, Chapman J, Brikci-Nigassa L, Sanhadji K, Touraine JL, Portoukalian J. Dendritic cells dysfunction in tumour environment. Cancer Lett. 2008. 272:186–196.
Article23. Bennaceur K, Chapman JA, Touraine JL, Portoukalian J. Immunosuppressive networks in the tumour environment and their effect in dendritic cells. Biochim Biophys Acta. 2009. 1795:16–24.
Article24. Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992. 356:607–609.
Article25. Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell Immunol. 1996. 170:111–119.26. Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006. 25:323–331.
Article27. Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998. 92:4778–4791.
Article28. Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004. 172:464–474.
Article29. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007. 25:267–296.
Article30. Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM Jr. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001. 61:363–369.31. Kanazawa M, Mori Y, Yoshihara K, Iwadate M, Suzuki S, Endoh Y, Ohki S, Takita K, Sekikawa K, Takenoshita S. Effect of PSK on the maturation of dendritic cells derived from human peripheral blood monocytes. Immunol Lett. 2004. 91:229–238.
Article32. Kim GY, Han MG, Song YS, Shin BC, Shin YI, Lee HJ, Moon DO, Lee CM, Kwak JY, Bae YS, Lee JD, Park YM. Proteoglycan isolated from Phellinus linteus induces toll-like receptors 2- and 4-mediated maturation of murine dendritic cells via activation of ERK, p38, and NF-kappaB. Biol Pharm Bull. 2004. 27:1656–1662.
Article33. Kim GY, Lee MY, Lee HJ, Moon DO, Lee CM, Jin CY, Choi YH, Jeong YK, Chung KT, Lee JY, Choi IH, Park YM. Effect of water-soluble proteoglycan isolated from Agaricus blazei on the maturation of murine bone marrow-derived dendritic cells. Int Immunopharmacol. 2005. 5:1523–1532.
Article34. Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006. 119:254–264.
Article35. Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996. 153:85–106.
Article36. Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998. 16:495–521.
Article37. Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007. 25:381–418.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nutritional and Physicochemical Characteristics of the Antidementia Acetylcholinesterase-Inhibiting Methanol Extracts from Umbilicaria esculenta
- Maturation of bone marrow-derived dendritic cells by a novel beta-glucan purified from Paenibacillus polymyxa JB115
- Cloning and Molecular Characterization of beta-1,3-Glucan Synthase from Sparassis crispa
- Immunostimulatory Effects of beta-glucan Purified from Paenibacillus polymyxa JB115 on Mouse Splenocytes
- Dectin-1 Stimulation Selectively Reinforces LPS-driven IgG1 Production by Mouse B Cells