Transl Clin Pharmacol.
2015 Jun;23(1):31-34. 10.12793/tcp.2015.23.1.31.
Assessment of statistical power for covariate effects in data from phase I clinical trials
- Affiliations
-
- 1Department of Pharmacology, Yonsei University College of Medicine, Seoul 120-752, Korea. kspark@yuhs.ac
- 2Brain Korea 21 Plus Project for Medical Science, Yonsei University, Seoul 120-752, Korea.
- 3Department of Clinical Pharmacology and Therapeutics, Inje University Pusan Paik Hospital, Busan 614-735, Korea.
- KMID: 2148857
- DOI: http://doi.org/10.12793/tcp.2015.23.1.31
Abstract
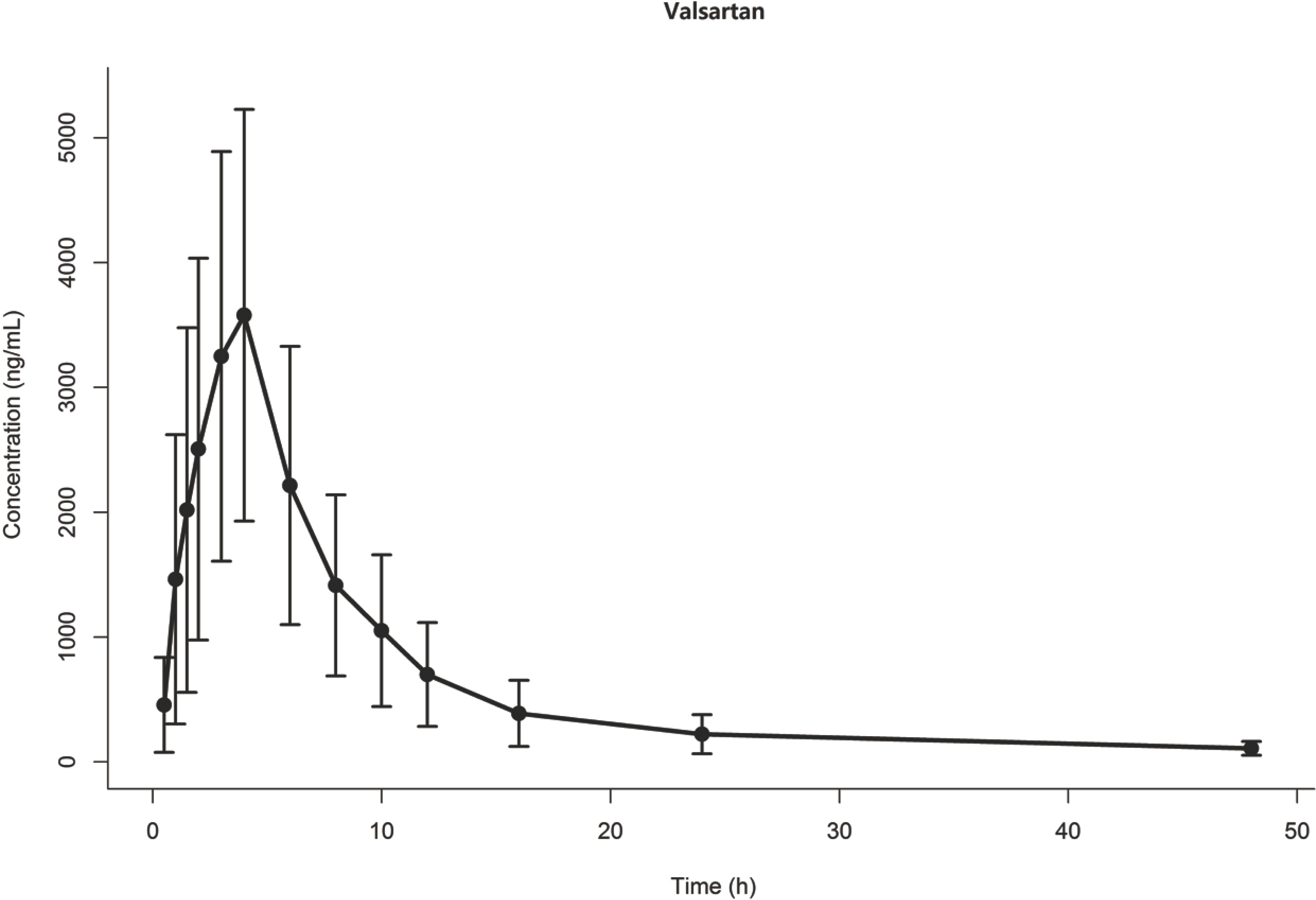
- One of the important purposes in population pharmacokinetic studies is to investigate the relationships between parameters and covariates to describe parameter variability. The purpose of this study is to evaluate the model's ability to correctly detect the parameter-covariate relationship that can be observed in phase I clinical trials. Data were simulated from a two-compartment model with zero-order absorption and first-order elimination, which was built from valsartan's concentration data collected from a previously conducted study. With creatinine clearance (CLCR) being used as a covariate to be tested, 3 different significance levels of 0.001
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wählby U, Jonsson EN, Karlsson MO. Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS PharmSci. 2002; 4:E27.
Article2. Analysis of clinical trial approval in 2014. http://www.mfds.go.kr/index. do?mid=675&seq=26363 Accessed 23 March. 2015.3. Kim Y, Son M, Lee D, Roh H, Son H, Chae D, et al. Pharmacokinetic comparison of 2 fixed-dose Combination tablets of amlodipine and valsartan in healthy male Korean volunteers: a randomized, open-label, 2-period, single-dose, crossover study. Clin Ther. 2013; 35:934–940. doi: 10.1016/j. clinthera.2013.05.021.
Article4. Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004; 75:85–94.
Article5. Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for nonlinear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005; 79:241–257.
Article6. Bauer RJ. INTRODUCTION TO NONMEM 7.3.0. NONMEM USERS GUIDE. Hanover. Maryland: ICON Development Solutions;2013.7. Ribbing J, Jonsson EN. Power, selection bias and predictive performance of the Population Pharmacokinetic Covariate Model. J Pharmacokinet Pharmacodyn. 2004; 31:109–134.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usage of Statistics in Clinical Trials
- The Effect of Increasing Control-to-case Ratio on Statistical Power in a Simulated Case-control SNP Association Study
- Sample size determination and power analysis using the G*Power software
- A Review on the Methods of Sample Size Determination in Nursing Research
- Comparative Effects and Safety of Full-Endoscopic Versus Microscopic Spinal Decompression for Lumbar Spinal Stenosis: A Meta-Analysis and Statistical Power Analysis of 6 Randomized Controlled Trials