J Cardiovasc Ultrasound.
2009 Jun;17(2):40-53. 10.4250/jcu.2009.17.2.40.
Role of Echocardiography in the Emergency Department
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. younhj@catholic.ac.kr
- KMID: 2135444
- DOI: http://doi.org/10.4250/jcu.2009.17.2.40
Abstract
- Echocardiography can play a key role in the diagnosis, evaluation and triage of patients presenting with acute chest pain in the emergency department, because of its rapid, accurate and repetitive image acquisition. Echocardiography can detect coronary artery disease, complications of acute myocardial infarction, pericardial emergency, acute aortic diseases, and pulmonary embolic events, all of which may cause acute chest pain. Depending on the clinical situation, stress echocardiography or contrast echocardiography may provide additional information. Ongoing technical development of imaging acquisition and analysis in echocardiography will increase its use in the differential diagnosis of acute chest pain.
Keyword
MeSH Terms
Figure
Reference
-
1. de Winter RJ, Koster RW, Sturk A, Sanders GT. Value of myoglobin, troponin T, and CK-Mb mass in ruling out an acute myocardial infarction in the emergency room. Circulation. 1995. 92:3401–3407.
Article2. Otto CM. Textbook of Clinical Echocardiography. 2004. 3rd edition. Philadelphia: Elsevier Saunders.3. Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davidson TW, Davis JL, Douglas PS, Gillam LD. ACC/AHA guidelines for the clinical application of echocardiography. A report of the American college of Cardiology/American heart association task force on practice guidelines (committee on clinical application of echocardiography). Developed in collaboration with the American society of echocardiography. Circulation. 1997. 95:1686–1744.
Article4. Goodkin GM, Spevack DM, Tunick PA, Kronzon I. How useful is hand carried bedside echocardiography in critically ill patients? J Am Coll Cardiol. 2001. 37:2019–2022.
Article5. Weston P, Alexander JH, Patel MR, Maynard C, Crawford L, Wagner GS. Hand-held echocardiographic examination of patients with symptoms of acute coronary syndromes in the emergency department: The 30-day outcome associated with normal left ventricular wall motion. Am Heart J. 2004. 148:1096–1101.
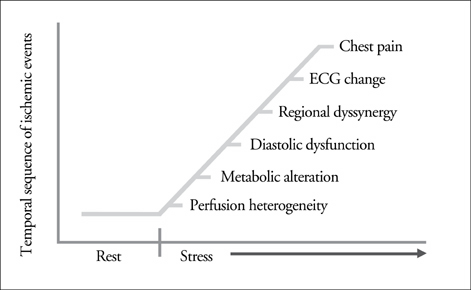
Article6. Hauser AM, Gangadharan V, Ramos RG, Gordon S, Timmis GC. Sequence of mechanical, electrocardiographic and clinical effects of repeated coronary artery occlusion in human beings: Echocardiographic observations during coronary angioplasty. J Am Coll Cardiol. 1985. 5:193–197.
Article7. Choi YS, Youn HJ, Jung SE, Choi YW, Lee DH, Park CS, Oh YS, Seung KB, Kim JH, Hong SJ. The association between coronary artery calcification on MDCT and angiographic coronary artery stenosis. Korean Circ J. 2007. 37:167–172.
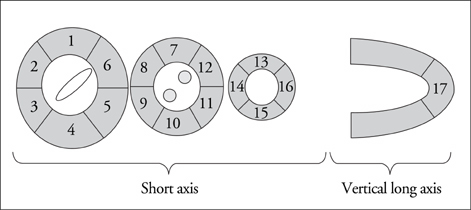
Article8. Schiller NB, Shah PM, Crawford M, De Maria A, Devereux R, Feigenbaum H, Gutgesell H, Reicheck N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989. 2:358–367.
Article9. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005. 18:1440–1463.
Article10. Oh JK, Gibbons RJ, Christian TF, Gersh BJ, Click RL, Sitthisook S, Tajik AJ, Seward JB. Correlation of regional wall motion abnormalities detected by two-dimensional echocardiography with perfusion defect determined by technetium 99m sestamibi imaging in patients treated with reperfusion therapy during acute myocardial infarction. Am Heart J. 1996. 131:32–37.
Article11. Youn HJ. Direct visualization of coronary artery and flow using transthoracic Doppler echocardiography. J Korean Pediatr Cardiol Soc. 2006. 10:354–366.12. Youn HJ. Demonstration of pathologic coronary flow dynamics using transthoracic Doppler echocardiography: Its potential role in clinical decision-making. Korean Circ J. 2005. 35:269–281.
Article13. Opherk D, Mall G, Zebe H, Schwarz F, Weihe E, Manthey J, Kübler W. Reduction of coronary reserve: A mechanism for angina pectoris in patients with arterial hypertension and normal coronary arteries. Circulation. 1984. 69:1–7.
Article14. Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE. Angina caused by reduced vasodilator reserve of the small coronary arteries. J Am Coll Cardiol. 1983. 1:1359–1373.
Article15. Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL. Decreased coronary flow reserve: A mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med. 1982. 307:1362–1366.16. Youn HJ, Park CS, Cho EJ, Chung HO, Jeon HK, Lee JM, Oh YS, Chung WS, Chae JS, Kim JH, Choi KB, Hong SJ. Clinical significance of slow flow velocity in the distal left anterior descending coronary artery detected by transthoracic Doppler ehocardiography. Korean Circ J. 2002. 32:299–308.
Article17. Watanabe N, Akasaka T, Yamaura Y, Akiyama M, Koyama Y, Kamiyama N, Neishi Y, Kaji S, Saito Y, Yoshida K. Noninvasive detection of total occlusion of the left anterior descending coronary artery with transthoracic Doppler echocardiography. J Am Coll Cardiol. 2001. 38:1328–1332.
Article18. Badruddin SM, Ahmad A, Mickelson J, Abukhalil J, Winters WL, Nagueh SF, Zoghbi WA. Supine bicycle versus posttreadmil exercise echocardiography in the detection of myocardial ischemia: A randomized single-blind crossover trial. J Am Coll Cardiol. 1999. 33:1485–1490.
Article19. Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: Techniques, implementation, clinical applications and correlations. Mayo Clin Proc. 1995. 70:5–15.
Article20. Quiñones MA, Verani MS, Haichin RM, Mahmarian JJ, Suarez J, Zoghbi WA. Exercise echocardiography versus 201Tl single-photon emission computed tomography in evaluation of coronary artery disease: Analysis of 292 patients. Circulation. 1992. 85:1026–1031.
Article21. Pellikka PA, Roger VL, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part II. Dobutamine stress echocardiography: Techniques, implementation, clinical applications and correlations. Mayo Clin Proc. 1995. 70:16–27.
Article22. Savonitto S, Ardissino D, Granger CB, Morando G, Prando MD, Mafrici A, Cavallini C, Melandri G, Thompson TD, Vahanian A, Ohman EM, Califf RM, Van de Werf F, Topol EJ. Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA. 1999. 281:707–713.
Article23. Rainbird AJ, Mulvagh SL, Oh JK, McCully RB, Klarich KW, Shub C, Mahoney DW, Pellikka PA. Contrast dobutamine stress echocardiography: Clinical practice assessment in 300 consecutive patients. J Am Soc Echocardiogr. 2001. 14:378–385.
Article24. Czitrom D, Karila-Cohen D, Brochet E, Juliard JM, Faraggi M, Aumont MC, Assayag P, Steg PG. Acute assessment of microvascular perfusion patterns by myocardial contrast echocardiography during myocardial infarction: Relation to timing and extent of functional recovery. Heart. 1999. 81:12–16.
Article25. Vançon AC, Fox ER, Chow CM, Hill J, Weyman AE, Picard MH, Scherrer-Crosbie M. Pulse inversion harmonic imaging improves endocardial border visualization in two-dimensional images: Comparison with harmonic imaging. J Am Soc Echocardiogr. 2002. 15:302–308.
Article26. Premawardhana U, Celermajer DS. Advances in echocardiography. Aust N Z J Med. 2000. 30:360–366.
Article27. Kang DH, Kang SJ, Song JM, Choi KJ, Hong MK, Song JK, Park SW, Park SJ. Efficacy of myocardial contrast echocardiography in the diagnosis and risk stratification of acute coronary syndrome. Am J Cardiol. 2005. 96:1498–1502.
Article28. Kaul S, Senior R, Firschke C, Wang XQ, Lindner J, Villanueva FS, Firozan S, Kontos MC, Taylor A, Nixon IJ, Watson DD, Harrell FE. Incremental value of cardiac imaging in patients presenting to the emergency department with chest pain and without ST-segment elevation: A multicenter study. Am Heart J. 2004. 148:129–136.
Article29. Hillis GS, Mulvagh SL, Gunda M, Hagen ME, Reeder GS, Oh JK. Contrast echocardiography using intravenous octafluoropropane and real-time perfusion imaging predicts functional recovery after myocardial infarction. J Am Soc Echocardiogr. 2003. 16:638–645.
Article30. Lepper W, Hoffmann R, Kamp O, Franke A, de Cock CC, Kühl HP, Sieswerda GT, Dahl J, Janssens U, Voci P, Visser CA, Hanrath P. Assessment of myocardial reperfusion by intravenous myocardial contrast echocardiography and coronary flow reverse after primary percutaneous transluminal angioplasty in patients with acute myocardial infarction. Circulation. 2000. 101:2368–2374.
Article31. Chirillo F, Cavarzerani A, Ius P, Totis O, Bruni A, Valfré C, Stritoni P. Role of transthoracic, transesophageal, and transgastric two-dimensional and color Doppler echocardiography in the evaluation of mechanical complications of acute myocardial infarction. Am J Cardiol. 1995. 76:833–836.
Article32. Lemery R, Smith HC, Giuliani ER, Gersh BJ. Prognosis in rupture of the ventricular septum after acute myocardial infarction and role of early surgical intervention. Am J Cardiol. 1992. 70:147–151.
Article33. Menon V, Webb JG, Hillis LD, Sleeper LA, Abboud R, Dzavik V, Slater JN, Forman R, Monrad ES, Talley JD, Hochman JS. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: A report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries in cardiogenic shock? J Am Coll Cardiol. 1984. 36:1080–1087.34. Kishon Y, Iqbal A, Oh JK, Gersh BJ, Freeman WK, Seward JB, Tajik AJ. Evolution of echocardiographic modalities in detection of post myocardial infarction ventricular septal defect and papillary muscle rupture: Study of 62 patients. Am Heart J. 1993. 126:667–675.
Article35. Purcaro A, Costantini C, Ciampani N, Mazzanti M, Silenzi C, Gili A, Belardinelli R, Astolfi D. Diagnostic criteria and management of subacute ventricular free wall rupture complicating acute myocardial infarction. Am J Cardiol. 1997. 80:397–405.
Article36. Yeo TC, Malouf JF, Oh JK, Seward JB. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann Intern Med. 1998. 128:299–305.
Article37. Goldstein JA. Pathophysiology and management of right heart ischemia. J Am Coll Cardiol. 2002. 40:841–853.
Article38. Ozdemir K, Altunkeser BB, Içli A, Ozdil H, Gök H. New parameters in identification of right ventricular myocardial infarction and proximal right coronary artery lesion. Chest. 2003. 124:219–226.
Article39. Esakof DD, Vannan MA, Pandian NG, Cao QL, Schwartz SL, Bojar RM. Visualization of left ventricular pseudoaneurysm with panoramic transesophageal echocardiography. J Am Soc Echocardiogr. 1994. 7:174–178.
Article40. Foster E, Schiller NB. The role of transesophageal echocardiography in critical care: UCSF experience. J Am Soc Echocardiogr. 1992. 5:368–374.
Article41. Widimsky P, Gregor P. Pericardial involvement during the course of myocardial infarction. A long-term clinical and echocardiographic study. Chest. 1995. 1081:89–93.42. Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, Bailey KR, Seward JB. Consecutive 1127 therapeutic echocardiographically guided pericardiocentesis: Clinical profile, practice pattern, and outcomes spanning 21 years. Mayo Clin Proc. 2002. 77:429–436.
Article43. Spodick DH. Acute cardiac tamponade. N Eng J Med. 2003. 349:684–690.
Article44. Appleton CP, Hatle LK, Popp RL. Cardiac tamponade and pericardial effusion: Respiratory variation in transvalvular flow velocities by Doppler echocardiography. J Am Coll Cardiol. 1988. 11:1020–1030.
Article45. O'Sullivan J, Heads A, Hunter S. Microbubble image enhancement and pericardiocentesis. Int J Cardiol. 1993. 42:95–96.46. Erbel R, Börner N, Steller D, Brunier J, Thelen M, Pfeiffer C, Mohr-Kahaly S, Iversen S, Oelert H, Meyer J. Detection of aortic dissection by transesophageal echocardiography. Br Heart J. 1987. 58:45–51.47. Khandheria BK, Tajik AJ, Taylor CL, Safford RE, Miller FA Jr, Stanson AW, Sinak LJ, Oh JK, Seward JB. Aortic dissection: Review of value and limitations of two-dimensional echocardiography in a six year experience. J Am Soc Echocardiogr. 1989. 2:17–24.
Article48. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): New insights into old disease. JAMA. 2000. 283:897–903.
Article49. Erbel R, Oelert H, Meyer J, Puth M, Mohr-Katoly S, Hausmann D, Daniel W, Maffei S, Caruso A, Covino FE. Effect of medical and surgical therapy on aortic dissection evaluated by transesophageal echocardiography. Implications for prognosis and therapy. Circulation. 1993. 87:1604–1615.
Article50. Kwon JA, Youn HJ, Oh YS, Choi SH, Lee JS, Lee MH, Chang JH, Park HS, Park CS, Lee JM, Chung WS, Hong SJ. A case of acute aortic dissection involved left and right coronary arterial ostia diagnosed with transesophageal echocardiography. J Korean Soc Echocardiogr. 2001. 9:141–145.
Article51. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year populationbased study. Arch Intern Med. 1998. 158:585–593.
Article52. Sinha N, Yalamanchili K, Sukhija R, Aronow WS, Fleisher AG, Maguire GP, Lehrman SG. Role of the 12-lead electrocardiogram in diagnosing pulmonary embolism. Cardiol Rev. 2005. 13:46–49.
Article53. Rodger M, Makropoulos D, Turek M, Quevillon J, Raymond F, Rasuli P, Wells PS. Diagnostic value of the electrocardiogram in suspected pulmonary embolism. Am J Cardiol. 2000. 86:807–809.
Article54. Kasper W, Geibel A, Tiede N, Bassenge D, Kauder E, Konstantinides S, Meinertz T, Just H. Distinguishing between acute and subacute massive pulmonary embolism by conventional and Doppler echocardiography. Br Heart J. 1993. 70:352–356.
Article55. Belenkie I, Dani R, Smith ER, Tyberg JV. Effects of volume loading during experimental acute pulmonary embolism. Circulation. 1989. 80:178–188.
Article56. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996. 78:469–473.
Article57. Proano M, Oh JK, Frye RL, Johnson CM, Tajik AJ, Taliercio CP. Successful treatment of pulmonary embolism and associated mobile right atrial thrombus with use of central thrombolytic infusion. Mayo Clin Proc. 1988. 63:1181–1185.
Article58. Torbicki A, Galié N, Covezzoli A, Rossi E, De Rosa M, Goldhaber SZ. ICOPER study group. Right heart thrombi in pulmonary embolism: Results from the international cooperative pulmonary embolism resistry. J Am Coll Cardiol. 2003. 41:2245–2251.59. Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: Right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997. 134:479–487.
Article60. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. National heart, lung, and blood institute working group on cellular and molecular mechanisms of right heart failure. Right ventricular function and failure. Circulation. 2006. 114:1883–1891.
Article61. Vannan Mani A. Atlas of echocardiography. 2005. 1st edition. Philadelphia: Current Medicine Group.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Echocardiography in Diagnosing Myocardial Ischemia at Emergency Department
- Transesophageal Echocardiography and Its Role in Intraoperative Echocardiography
- Role of Echocardiography in Small Animal Research
- Predicting Factors for Cardiovascular Injuries and and Indication for Emergency Echocardiography in Sternal Fractures
- Thrombi in Main Pulmonary Artery Detected by Echocardiography