J Korean Med Sci.
2015 Mar;30(3):317-322. 10.3346/jkms.2015.30.3.317.
Comparison of Pathological and Biochemical Outcomes after Radical Prostatectomy in Korean Patients with Serum PSA Ranges
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. besthml@medimail.co.kr
- KMID: 2133311
- DOI: http://doi.org/10.3346/jkms.2015.30.3.317
Abstract
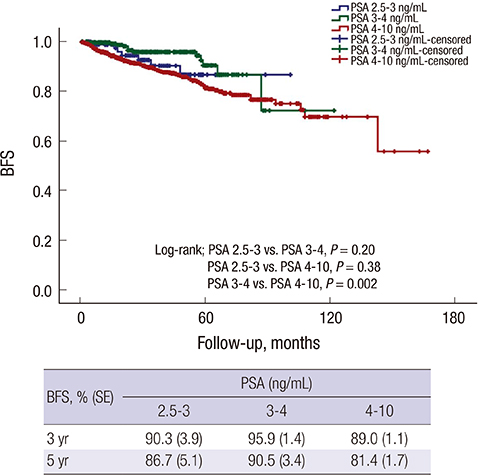
- The aim of this study was to assess surgical outcome at radical prostatectomy (RP) in Korean men with a serum prostate-specific antigen (PSA) level of 2.5 to 3.0 ng/mL and compared with those of patients who had a PSA level of 3.0-4.0 and 4.0-10.0 ng/mL. We retrospectively compared clinico-pathological characteristics and biochemical recurrence (BCR) risk in patients with PSA level of 2.5-3.0 (group 1, n = 92, 5.7%), 3.0-4.0 (group 2, n = 283, 17.5%), or 4.0-10.0 ng/mL (group 3, n = 1,242, 76.8%) who underwent RP between 1995 and 2013. The pathologic characteristics including Gleason score, pathologic stage, and percentage of significant cancer in group 1 were similar to those in group 2 and group 3. Furthermore, pathological upgrading and upstaging were found in 23 (30.7%) and 10 (14.7%) in group 1, 84 (33.9%) and 19 (8.8%) in group 2, and 321 (32.8%) and 113 (12.8%) in group 3, respectively, with no significant differences among the three groups (all P > 0.05). In multivariate analysis, PSA grouping was not an independent predictor of BCR. Within the population with PSA lower than 10 ng/mL, substratification of PSA is not a significant predictor for upgrading, upstaging, or adverse prognosis.
MeSH Terms
Figure
Reference
-
1. Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012; 61:1079–1092.2. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010; 25:1113–1121.3. Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010; 8:145.4. Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, Pihl CG, Stranne J, Holmberg E, Lilja H. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010; 11:725–732.5. Wolf JS Jr. 2012 AUA clinical practice guidelines. Introduction. J Urol. 2012; 188:2454.6. Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004; 350:2239–2246.7. Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schröder FH, de Koning HJ. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003; 95:868–878.8. Loeb S, Roehl KA, Helfand BT, Catalona WJ. Complications of open radical retropubic prostatectomy in potential candidates for active monitoring. Urology. 2008; 72:887–891.9. Kobayashi T, Nishizawa K, Ogura K, Mitsumori K, Ide Y. Detection of prostate cancer in men with prostate-specific antigen levels of 2.0 to 4.0 ng/mL equivalent to that in men with 4.1 to 10.0 ng/mL in a Japanese population. Urology. 2004; 63:727–731.10. Park HK, Hong SK, Byun SS, Lee SE. T1c prostate cancer detection rate and pathologic characteristics: comparison between patients with serum prostate-specific antigen range of 3.0 to 4.0 ng/mL and 4.1 to 10.0 ng/mL in Korean population. Urology. 2006; 68:85–88.11. Song C, Ro JY, Lee MS, Hong SJ, Chung BH, Choi HY, Lee SE, Lee E, Kim CS, Ahn H. Prostate cancer in Korean men exhibits poor differentiation and is adversely related to prognosis after radical prostatectomy. Urology. 2006; 68:820–824.12. Byun SS, Lee S, Lee SE, Lee E, Seo SI, Lee HM, Choi HY, Song C, Ahn H, Choi YD, et al. Recent changes in the clinicopathologic features of Korean men with prostate cancer: a comparison with Western populations. Yonsei Med J. 2012; 53:543–549.13. Kang DI, Chung JI, Ha HK, Min K, Yoon J, Kim W, Seo WI, Kang P, Jung SJ, Kim IY. Korean prostate cancer patients have worse disease characteristics than their American counterparts. Asian Pac J Cancer Prev. 2013; 14:6913–6917.14. Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RC, Hoedemaeker RF, van Leenders GJ, Schroder FH, van der Kwast TH. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011; 185:121–125.15. Park KK, Lee SH, Choi YD, Chung BH. Optimal baseline prostate-specific antigen level to distinguish risk of prostate cancer in healthy men between 40 and 69 years of age. J Korean Med Sci. 2012; 27:40–45.16. Chung MS, Lee SH, Lee DH, Kim SJ, Kim CS, Lee KS, Jung JI, Kim SW, Lee YS, Chung BH. Practice patterns of Korean urologists for screening and managing prostate cancer according to PSA level. Yonsei Med J. 2012; 53:1136–1141.17. Smith DS, Carvalhal GF, Mager DE, Bullock AD, Catalona WJ. Use of lower prostate specific antigen cutoffs for prostate cancer screening in black and white men. J Urol. 1998; 160:1734–1738.18. Sokoloff MH, Yang XJ, Fumo M, Mhoon D, Brendler CB. Characterizing prostatic adenocarcinomas in men with a serum prostate specific antigen level of < 4.0 ng/mL. BJU Int. 2004; 93:499–502.19. Pepe P, Panella P, D'Arrigo L, Savoca F, Pennisi M, Aragona F. Should men with serum prostate-specific antigen < or =4 ng/ml and normal digital rectal examination undergo a prostate biopsy? A literature review. Oncology. 2006; 70:81–89.20. Lughezzani G, Briganti A, Karakiewicz PI, Kattan MW, Montorsi F, Shariat SF, Vickers AJ. Predictive and prognostic models in radical prostatectomy candidates: a critical analysis of the literature. Eur Urol. 2010; 58:687–700.21. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005; 293:2095–2101.22. Mian BM, Lehr DJ, Moore CK, Fisher HA, Kaufman RP Jr, Ross JS, Jennings TA, Nazeer T. Role of prostate biopsy schemes in accurate prediction of Gleason scores. Urology. 2006; 67:379–383.23. Fitzpatrick JM, Banu E, Oudard S. Prostate-specific antigen kinetics in localized and advanced prostate cancer. BJU Int. 2009; 103:578–587.24. Corcoran NM, Casey RG, Hong MK, Pedersen J, Connolly S, Peters J, Harewood L, Gleave ME, Costello AJ, Hovens CM, et al. The ability of prostate-specific antigen (PSA) density to predict an upgrade in Gleason score between initial prostate biopsy and prostatectomy diminishes with increasing tumour grade due to reduced PSA secretion per unit tumour volume. BJU Int. 2012; 110:36–42.25. Sokoll LJ, Sanda MG, Feng Z, Kagan J, Mizrahi IA, Broyles DL, Partin AW, Srivastava S, Thompson IM, Wei JT, et al. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [-2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev. 2010; 19:1193–1200.26. Radwan MH, Yan Y, Luly JR, Figenshau RS, Brandes SB, Bhayani SB, Bullock AD, Liefu Y, Andriole GL, Kibel AS. Prostate-specific antigen density predicts adverse pathology and increased risk of biochemical failure. Urology. 2007; 69:1121–1127.27. Kunz I, Musch M, Roggenbuck U, Klevecka V, Kroepfl D. Tumour characteristics, oncological and functional outcomes in patients aged >/= 70 years undergoing radical prostatectomy. BJU Int. 2013; 111:E24–E29.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Predictors of Biochemical Failure after Radical Perineal Prostatectomy for Localized Prostate Cancer
- Use of Serum PSA in Comparison of Biopsy Gleason Score with Radical Prostatectomy Gleason Score
- Influences of Neoadjuvant Androgen Ablation before Radical Prostatectomy on Positive Surgical Margin and Biochemical Recurrence Rate
- Outcome and Prognostic Factors of Salvage Radiotherapy for Biochemical Failure after Radical Prostatectomy: A Single Institute Experience
- Salvage Radiotherapy for Patients with PSA Relapse Following Radical Prostatectomy: Issues and Challenges