J Korean Med Sci.
2015 Mar;30(3):252-258. 10.3346/jkms.2015.30.3.252.
Is Intravesical Bacillus Calmette-Guerin Therapy Superior to Chemotherapy for Intermediate-risk Non-muscle-invasive Bladder Cancer?: An Ongoing Debate
- Affiliations
-
- 1Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. cskim@amc.seoul.kr
- KMID: 2133301
- DOI: http://doi.org/10.3346/jkms.2015.30.3.252
Abstract
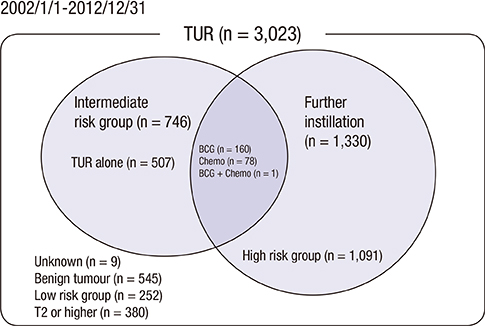
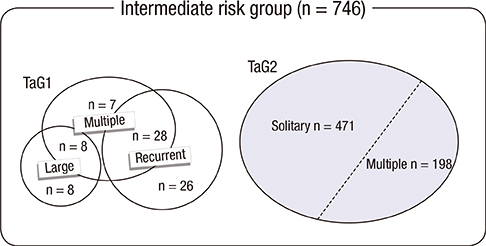
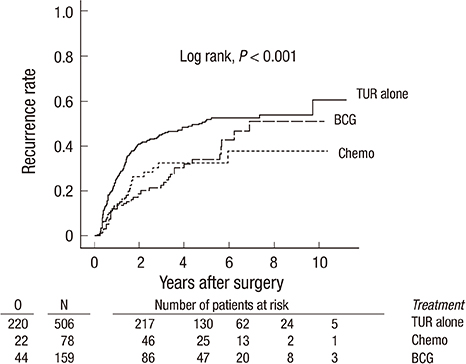
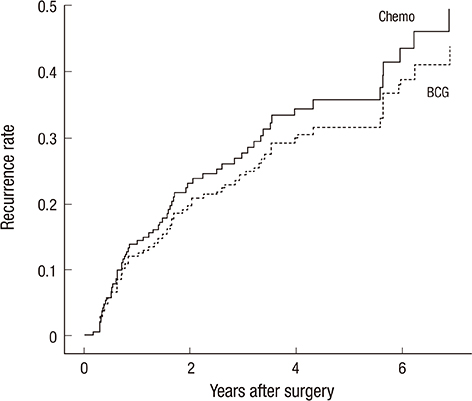
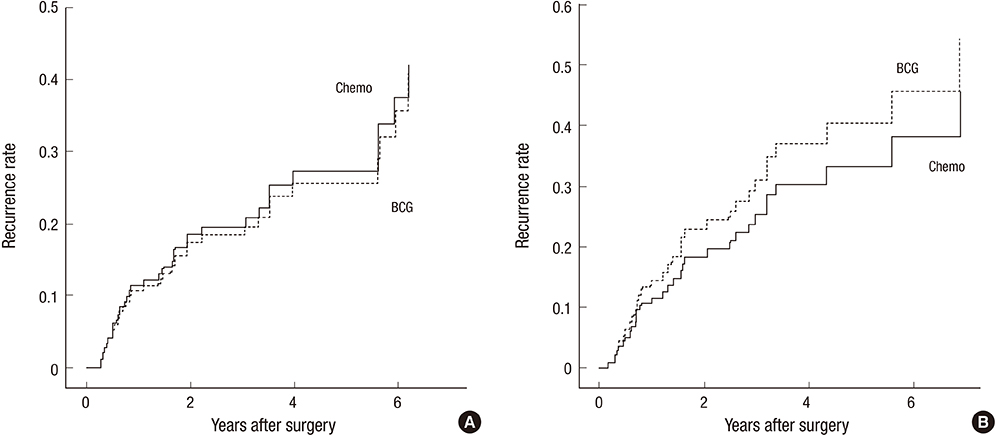
- The objective of this study was to evaluate the risk of recurrence in patients with intermediate-risk non-muscle-invasive bladder cancer (NMIBC) after intravesical instillation with chemotherapeutic agents or Bacillus Calmette-Guerin (BCG) therapy. A cohort of 746 patients with intermediate-risk NMIBC comprised the study group. The primary outcome was time to first recurrence. The recurrence rates of the transurethral resection (TUR) alone, chemotherapy, and BCG groups were determined using Kaplan-Meier analysis. Risk factors for recurrence were identified using Cox regression analysis. In total, 507 patients (68.1%), 78 patients (10.5%), and 160 (21.4%) underwent TUR, TUR+BCG, or TUR+chemotherapy, respectively. After a median follow-up period of 51.7 months (interquartile range=33.1-77.8 months), 286 patients (38.5%) developed tumor recurrence. The 5-yr recurrence rates for the TUR, chemotherapy, and BCG groups were 53.6%+/-2.7%, 30.8%+/-5.7%, and 33.6%+/-4.7%, respectively (P<0.001). Chemotherapy and BCG treatment were found to be predictors of reduced recurrence. Cox-regression analysis showed that TUR+BCG did not differ from TUR+chemotherapy in terms of recurrence risk. Adjuvant intravesical instillation is an effective prophylactic that prevents tumor recurrence in intermediate-risk NMIBC patients following TUR. In addition, both chemotherapeutic agents and BCG demonstrate comparable efficacies for preventing recurrence.
MeSH Terms
-
Adjuvants, Immunologic/*therapeutic use
Administration, Intravesical
Antineoplastic Agents/*therapeutic use
BCG Vaccine/*therapeutic use
Female
Follow-Up Studies
Humans
Male
Middle Aged
Neoplasm Recurrence, Local/*pathology
Neoplasm Staging
Risk
Treatment Outcome
Urinary Bladder/pathology
Urinary Bladder Neoplasms/*drug therapy/pathology/surgery
Adjuvants, Immunologic
Antineoplastic Agents
BCG Vaccine
Figure
Reference
-
1. Oosterlinck W, Solsona E, Akaza H, Busch C, Goebell PJ, Malmstrom PU, Ozen H, Sved P. Low-grade Ta (noninvasive) urothelial carcinoma of the bladder. Urology. 2005; 66:75–89.2. Holmang S, Johansson SL. Stage Ta-T1 bladder cancer: the relationship between findings at first followup cystoscopy and subsequent recurrence and progression. J Urol. 2002; 167:1634–1637.3. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006; 49:466–465.4. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J, Rouprêt M. European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011; 59:997–1008.5. Brausi M, Witjes JA, Lamm D, Persad R, Palou J, Colombel M, Buckley R, Soloway M, Akaza H, Böhle A. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol. 2011; 186:2158–2167.6. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Bohle A, Palou Redorta J, et al. European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013; 64:639–653.7. Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, Gontero P, Hoeltl W, Turkeri L, Marreaud S, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013; 63:462–472.8. Witjes JA, Palou J, Soloway M, Lamm D, Kamat AM, Brausi M, Persad R, Buckley R, Colombel M, Böhle A. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): results of an international individual patient data survey (IPDS). BJU Int. 2013; 112:742–750.9. Shelley MD, Kynaston H, Court J, Wilt TJ, Coles B, Burgon K, Mason MD. A systematic review of intravesical bacillus Calmette-Guerin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001; 88:209–216.10. Han RF, Pan JG. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006; 67:1216–1223.11. Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003; 169:90–95.12. Sylvester RJ, van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002; 168:1964–1970.13. Böhle A, Bock PR. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004; 63:682–686.14. Sylvester RJ, Brausi MA, Kirkels WJ, Hoeltl W, Calais Da Silva F, Powell PH, Prescott S, Kirkali Z, van de Beek C, Gorlia T, et al. EORTC Genito-Urinary Tract Cancer Group. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010; 57:766–773.15. Malmstrom PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, Solsona E, Di Stasi SM, Witjes JA. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009; 56:247–256.16. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001; 10:19–28.17. Grimm MO, Steinhoff C, Simon X, Spiegelhalder P, Ackermann R, Vogeli TA. Effect of routine repeat transurethral resection for superficial bladder cancer: a long-term observational study. J Urol. 2003; 170:433–437.18. Schwaibold HE, Sivalingam S, May F, Hartung R. The value of a second transurethral resection for T1 bladder cancer. BJU Int. 2006; 97:1199–1201.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal Management of Bacillus Calmette-Guérin–Refractory Non–Muscle-Invasive Bladder Cancer in 2023
- New Drugs for Bacillus Calmette Guérin-Unresponsive Nonmuscle Invasive Bladder Cancer
- Emerging treatments for bacillus Calmette– Guérin-unresponsive non-muscle-invasive bladder cancer
- Treatment Case of Asymptomatic Prostate Tuberculosis That Developed After Bacillus Calmette-Guerin Intravesical Therapy in a Patient With Nonmuscle Invasive Bladder Cancer
- Tuberculous Prostatic Abscess Following Intravesical Bacillus Calmette-Guerin Instillation