Cancer Res Treat.
2015 Apr;47(2):306-312. 10.4143/crt.2014.015.
CCL2 Chemokine as a Potential Biomarker for Prostate Cancer: A Pilot Study
- Affiliations
-
- 1Department of Urology and Pediatric Urology, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany. igor.tsaur@kgu.de
- 2Department of Surgery, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany.
- 3Department of Surgery I, University Hospital Wuerzburg, Julius-Maximilians-University, Wuerzburg, Germany.
- KMID: 2132810
- DOI: http://doi.org/10.4143/crt.2014.015
Abstract
- PURPOSE
Prostate specific antigen is not reliable in diagnosing prostate cancer (PCa), making the identification of novel, precise diagnostic biomarkers important. Since chemokines are associated with more aggressive disease and poor prognosis in diverse malignancies, we aimed to investigate the diagnostic relevance of chemokines in PCa.
MATERIALS AND METHODS
Preoperative and early postoperative serum samples were obtained from 39 consecutive PCa patients undergoing radical prostatectomy. Serum from 15 healthy volunteers served as controls. Concentrations of CXCL12, CXCL13, CX3CL1, CCL2, CCL5, and CCL20 were measured in serum by Luminex. The expression activity of CXCR3, CXCR4, CXCR5, CXCR7, CXCL12, CXCL13, CX3CR1, CXCL1, CCR2, CCR5, CCR6, CCR7, CCL2, and CCL5 mRNA was assessed in tumor and adjacent normal tissue of prostatectomy specimens by quantitative real-time polymerase chain reaction. The associations of these chemokines with clinical and histological parameters were tested.
RESULTS
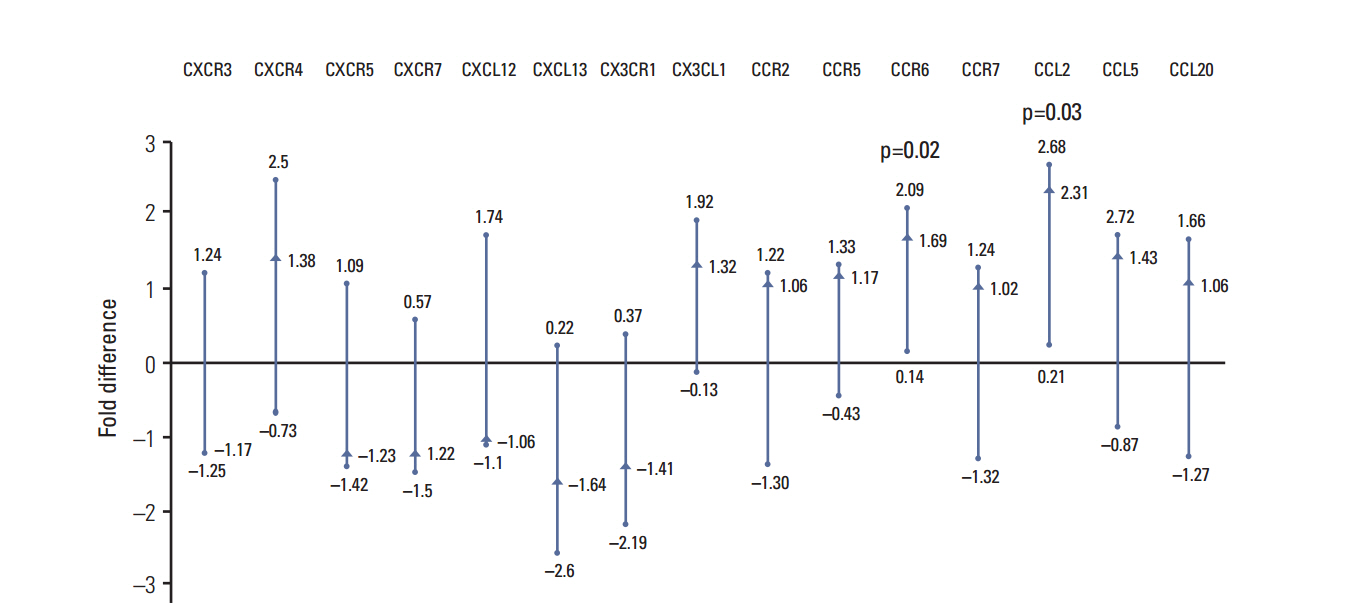
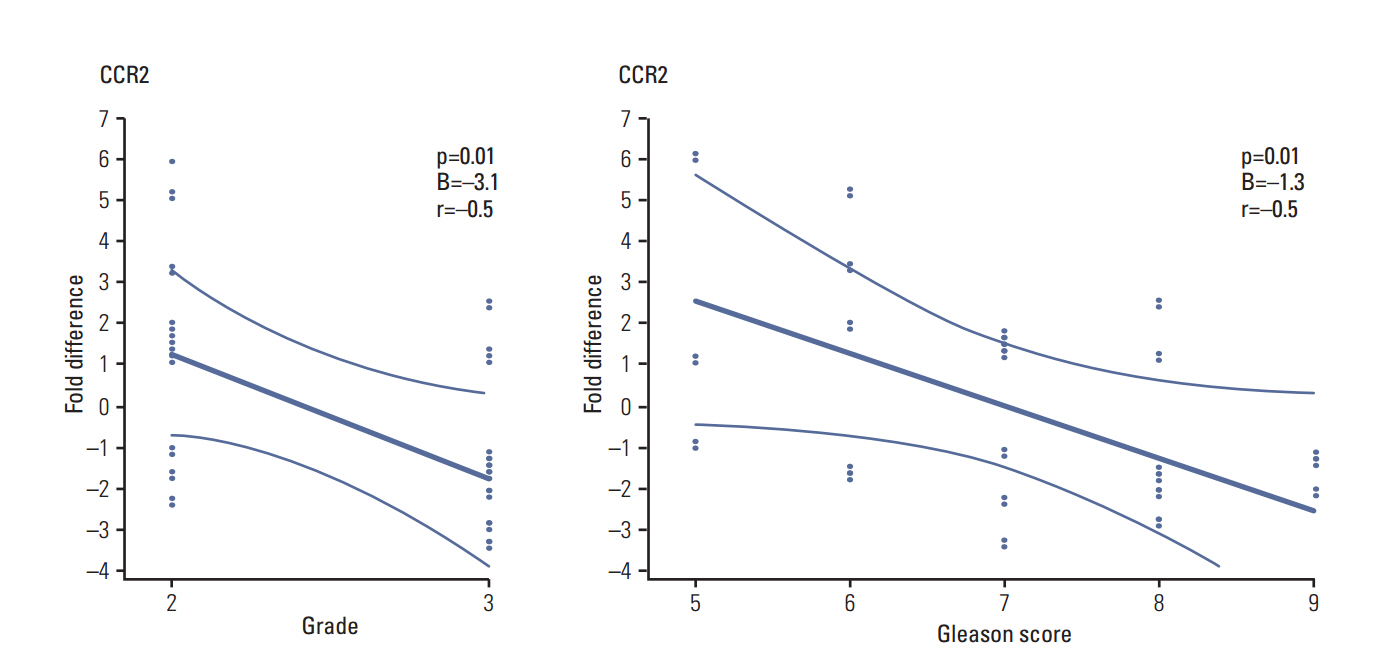
The gene expression activity of CCL2 and CCR6 was significantly higher in tumor tissue compared to adjacent normal tissue. CCL2 was also significantly higher in the blood samples of PCa patients, compared to controls. CCL5, CCL20, and CX3CL1 were lower in patient serum, compared to controls. CCR2 tissue mRNA was negatively correlated with the Gleason score and grading.
CONCLUSION
Chemokines are significantly modified during tumorigenesis of PCa, and CCL2 is a promising diagnostic biomarker.
MeSH Terms
-
Biological Markers
Carcinogenesis
Chemokine CCL2*
Chemokines
Diagnosis
Gene Expression
Healthy Volunteers
Humans
Neoplasm Grading
Passive Cutaneous Anaphylaxis
Pilot Projects*
Prognosis
Prostate-Specific Antigen
Prostatectomy
Prostatic Neoplasms*
Real-Time Polymerase Chain Reaction
RNA, Messenger
Biological Markers
Chemokine CCL2
Chemokines
Prostate-Specific Antigen
RNA, Messenger
Figure
Reference
-
References
1. Gumulec J, Sochor J, Hlavna M, Sztalmachova M, Krizkova S, Babula P, et al. Caveolin-1 as a potential high-risk prostate cancer biomarker. Oncol Rep. 2012; 27:831–41.2. Principe S, Jones EE, Kim Y, Sinha A, Nyalwidhe JO, Brooks J, et al. In-depth proteomic analyses of exosomes isolated from expressed prostatic secretions in urine. Proteomics. 2013; 13:1667–71.
Article3. Shen M, Chen W, Yu K, Chen Z, Zhou W, Lin X, et al. The diagnostic value of PCA3 gene-based analysis of urine sediments after digital rectal examination for prostate cancer in a Chinese population. Exp Mol Pathol. 2011; 90:97–100.
Article4. Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. Chemokines and chemokine receptors: an overview. Front Biosci (Landmark Ed). 2009; 14:540–51.
Article5. Lin TH, Liu HH, Tsai TH, Chen CC, Hsieh TF, Lee SS, et al. CCL2 increases alphavbeta3 integrin expression and subsequently promotes prostate cancer migration. Biochim Biophys Acta. 2013; 1830:4917–27.6. Craig MJ, Loberg RD. CCL2 (monocyte chemoattractant protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2006; 25:611–9.
Article7. Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002; 2:175–84.
Article8. Kulbe H, Levinson NR, Balkwill F, Wilson JL. The chemokine network in cancer--much more than directing cell movement. Int J Dev Biol. 2004; 48:489–96.9. Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004; 14:181–5.
Article10. Strieter RM, Belperio JA, Phillips RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004; 14:195–200.
Article11. Wedel SA, Raditchev IN, Jones J, Juengel E, Engl T, Jonas D, et al. CXC chemokine mRNA expression as a potential diagnostic tool in prostate cancer. Mol Med Rep. 2008; 1:257.12. Wittekind C, Meyer HJ, Bootz F. UICC: TNM Klassifikation maligner Tumoren. 6. Auflage. Berlin: Springer;2002.13. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005; 29:1228–42.
Article14. Waugh DJ, Wilson C, Seaton A, Maxwell PJ. Multi-faceted roles for CXC-chemokines in prostate cancer progression. Front Biosci. 2008; 13:4595–604.
Article15. Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007; 98:1652–8.
Article16. Tsaur I, Noack A, Waaga-Gasser AM, Makarevic J, Schmitt L, Kurosch M, et al. Chemokines involved in tumor promotion and dissemination in patients with renal cell cancer. Cancer Biomark. 2011; 10:195–204.
Article17. Gorlov IP, Sircar K, Zhao H, Maity SN, Navone NM, Gorlova OY, et al. Prioritizing genes associated with prostate cancer development. BMC Cancer. 2010; 10:599.
Article18. Izhak L, Wildbaum G, Weinberg U, Shaked Y, Alami J, Dumont D, et al. Predominant expression of CCL2 at the tumor site of prostate cancer patients directs a selective loss of immunological tolerance to CCL2 that could be amplified in a beneficial manner. J Immunol. 2010; 184:1092–101.
Article19. Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, et al. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006; 66:1311–8.
Article20. Shirotake S, Miyajima A, Kosaka T, Tanaka N, Kikuchi E, Mikami S, et al. Regulation of monocyte chemoattractant protein-1 through angiotensin II type 1 receptor in prostate cancer. Am J Pathol. 2012; 180:1008–16.
Article21. Agarwal M, He C, Siddiqui J, Wei JT, Macoska JA. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate. 2013; 73:573–81.
Article22. Ghadjar P, Loddenkemper C, Coupland SE, Stroux A, Noutsias M, Thiel E, et al. Chemokine receptor CCR6 expression level and aggressiveness of prostate cancer. J Cancer Res Clin Oncol. 2008; 134:1181–9.
Article23. Blum DL, Koyama T, M'Koma AE, Iturregui JM, Martinez-Ferrer M, Uwamariya C, et al. Chemokine markers predict biochemical recurrence of prostate cancer following prostatectomy. Clin Cancer Res. 2008; 14:7790–7.
Article24. Wang Q, Diao X, Sun J, Chen Z. Stromal cell-derived factor-1 and vascular endothelial growth factor as biomarkers for lymph node metastasis and poor cancer-specific survival in prostate cancer patients after radical prostatectomy. Urol Oncol. 2013; 31:312–7.
Article25. Macoska JA, Begley LA, Dunn RL, Siddiqui J, Wei JT, Sarma AV. Pilot and feasibility study of serum chemokines as markers to distinguish prostatic disease in men with low total serum PSA. Prostate. 2008; 68:442–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical characteristics of Monocyte chemotactic protein-1 in the Endometrium of women with Endometriosis
- Effect of Salicylate on the Monocyte Chemoattractant Protein-1 Expression and Intracellular Reactive Oxygen Species Formation in Human Mesangial Cells
- Effects of Monocyte Chemoattractant Protein-1 on Growth and Migration of Cultured Human Vascular Smooth Muscle Cells
- Chemokine Systems Link Obesity to Insulin Resistance
- Chemokine and Chemokine Receptor Polymorphisms in Bipolar Disorder