Cancer Res Treat.
2015 Apr;47(2):242-250. 10.4143/crt.2014.066.
Novel Methods for Clinical Risk Stratification in Patients with Colorectal Liver Metastases
- Affiliations
-
- 1Oral Cancer Research Institute, Seoul, Korea.
- 2Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Oral and Maxillofacial Surgery, Yonsei University College of Dentistry, Seoul, Korea.
- 4Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. ssj338@yuhs.ac
- 5Department of Diagnostic Radiology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2132802
- DOI: http://doi.org/10.4143/crt.2014.066
Abstract
- PURPOSE
Colorectal cancer patients with liver-confined metastases are classified as stage IV, but their prognoses can differ from metastases at other sites. In this study, we suggest a novel method for risk stratification using clinically effective factors.
MATERIALS AND METHODS
Data on 566 consecutive patients with colorectal liver metastasis (CLM) between 1989 and 2010 were analyzed. This analysis was based on principal component analysis (PCA).
RESULTS
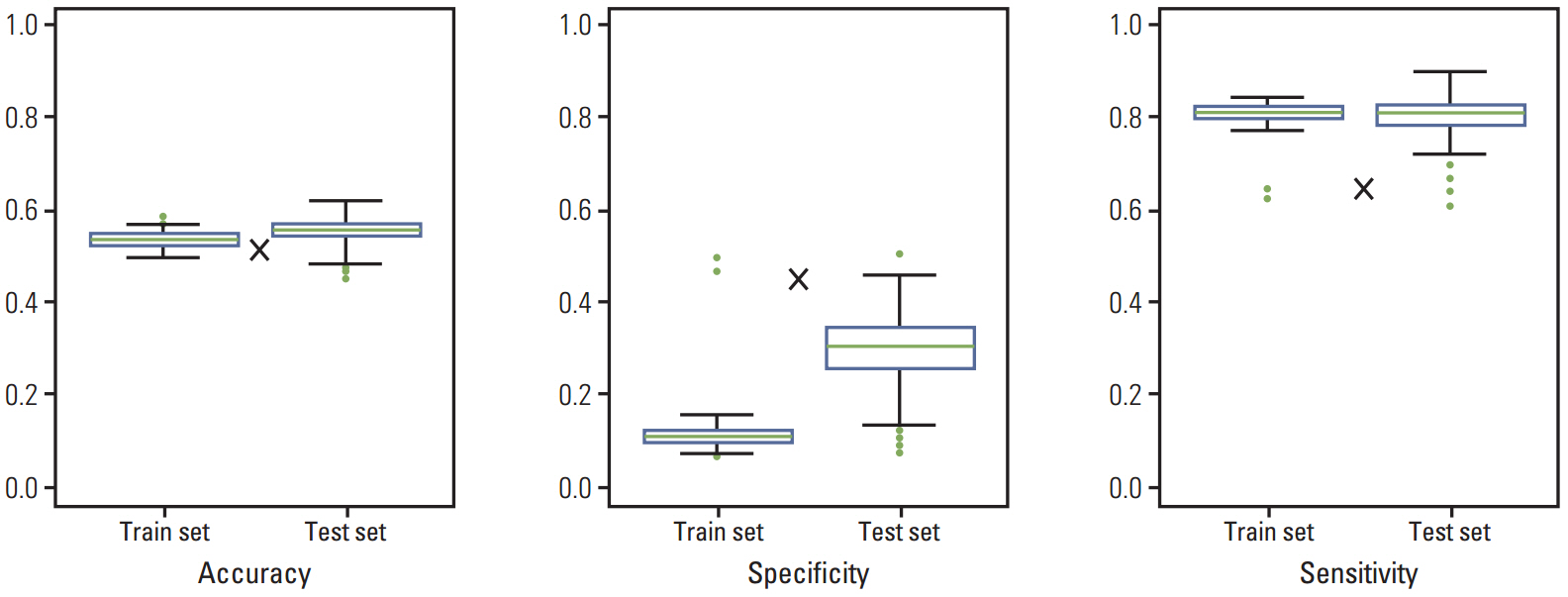
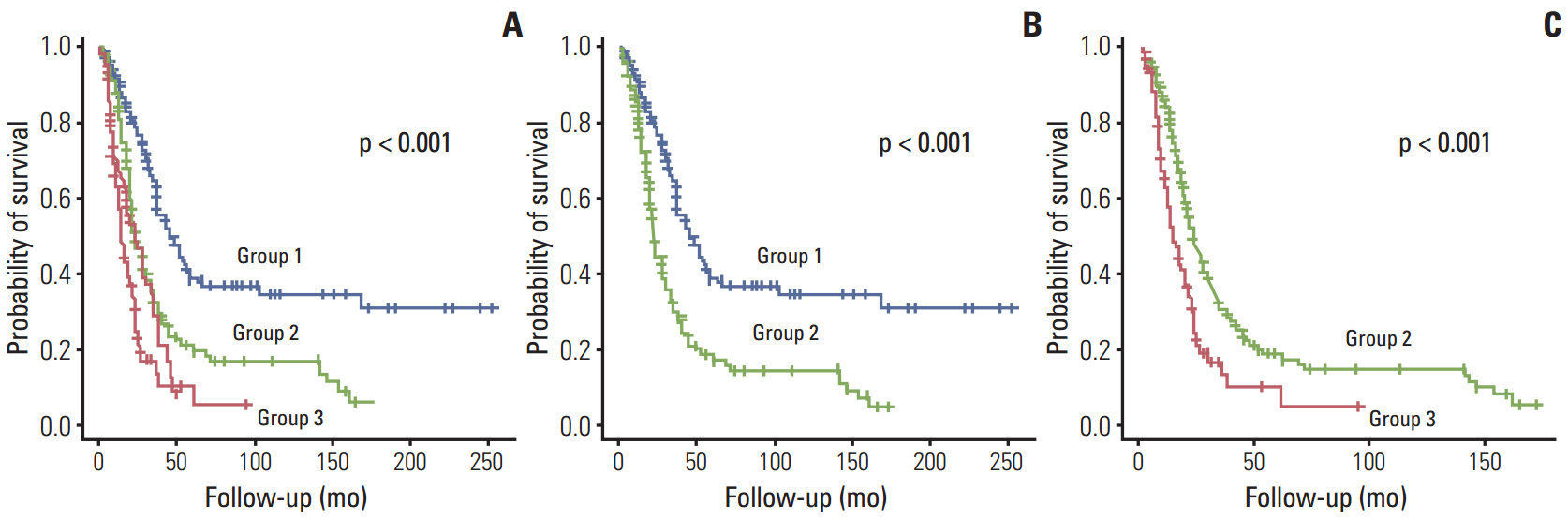
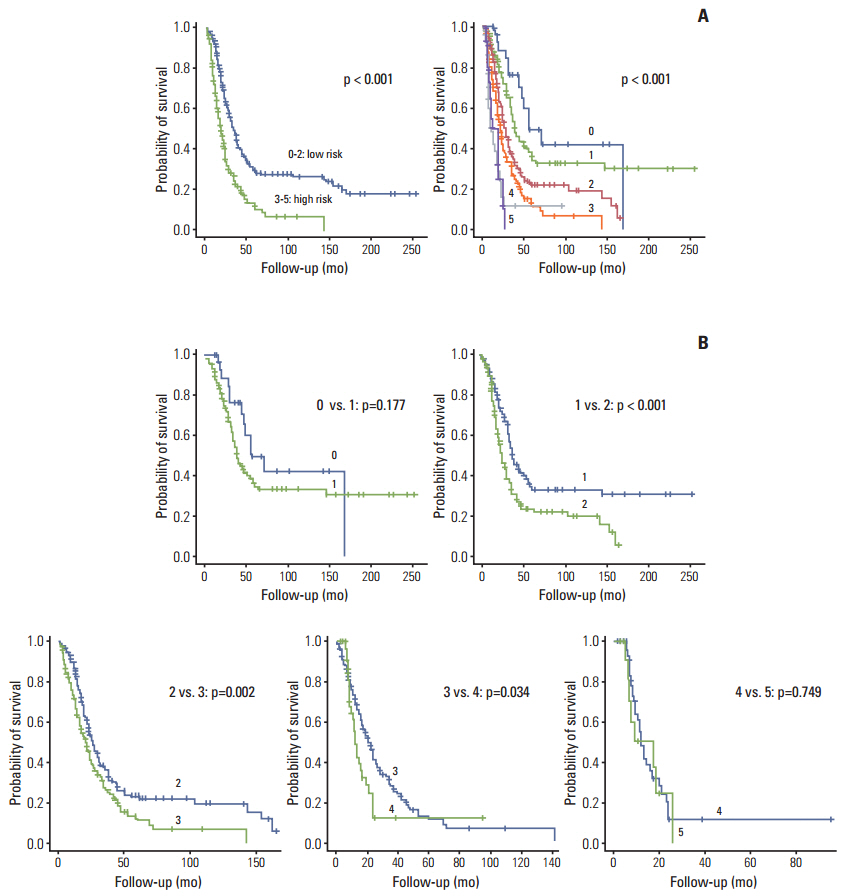
The survival rate was affected by carcinoembryonic antigen (CEA) level (p < 0.001; risk ratio, 1.90), distribution of liver metastasis (p=0.014; risk ratio, 1.46), and disease-free interval (DFI; p < 0.001; risk ratio, 1.98). When patients were divided into three groups according to PCA score using significantly affected factors, they showed significantly different survival patterns (p < 0.001).
CONCLUSION
The PCA scoring system based on CEA level, distribution of liver metastasis, and DFI may be useful for preoperatively determining prognoses in order to assist in clinical decisionmaking and designing future clinical trials for CLM treatment.
MeSH Terms
Figure
Cited by 1 articles
-
Preclinical Efficacy of [V4Q5]dDAVP, a Second Generation Vasopressin Analog, on Metastatic Spread and Tumor-Associated Angiogenesis in Colorectal Cancer
Juan Garona, Natasha T. Sobol, Marina Pifano, Valeria I. Segatori, Daniel E. Gomez, Giselle V. Ripoll, Daniel F. Alonso
Cancer Res Treat. 2019;51(2):438-450. doi: 10.4143/crt.2018.040.
Reference
-
References
1. Adam R, Hoti E, Folprecht G, Benson AB. Accomplishments in 2008 in the management of curable metastatic colorectal cancer. Gastrointest Cancer Res. 2009; 3(5 Suppl 2):S15–22.2. Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994; 343:1405–10.
Article3. Mella J, Biffin A, Radcliffe AG, Stamatakis JD, Steele RJ. Population-based audit of colorectal cancer management in two UK health regions. Colorectal Cancer Working Group, Royal College of Surgeons of England Clinical Epidemiology and Audit Unit. Br J Surg. 1997; 84:1731–6.4. Poston GJ, Adam R, Alberts S, Curley S, Figueras J, Haller D, et al. OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005; 23:7125–34.
Article5. Rees M, John TG. Current status of surgery in colorectal metastases to the liver. Hepatogastroenterology. 2001; 48:341–4.6. Papadimitriou JD, Fotopoulos AC, Prahalias AA, Vassiliou JG, Papadimitriou LJ. The impact of new technology on hepatic resection for malignancy. Arch Surg. 2001; 136:1307–13.
Article7. Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001; 8:347–53.8. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229–37.
Article9. Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004; 15:933–9.
Article10. Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995; 19:59–71.
Article11. Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996; 77:1254–62.12. Taylor M, Forster J, Langer B, Taylor BR, Greig PD, Mahut C. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg. 1997; 173:467–71.
Article13. Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999; 189:291–9.14. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002; 235:759–66.
Article15. Ambiru S, Miyazaki M, Isono T, Ito H, Nakagawa K, Shimizu H, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999; 42:632–9.16. Gomez D, Cameron IC. Prognostic scores for colorectal liver metastasis: clinically important or an academic exercise? HPB (Oxford). 2010; 12:227–38.
Article17. Welsh FK, Tekkis PP, John TG, Rees M. Predictive models in colorectal liver metastases--can we personalize treatment and outcome? Dig Surg. 2008; 25:406–12.18. Merkel S, Bialecki D, Meyer T, Muller V, Papadopoulos T, Hohenberger W. Comparison of clinical risk scores predicting prognosis after resection of colorectal liver metastases. J Surg Oncol. 2009; 100:349–57.
Article19. Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, et al. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009; 16:3279–88.
Article20. Fong Y, Salo J. Surgical therapy of hepatic colorectal metastasis. Semin Oncol. 1999; 26:514–23.
Article21. Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004; 139:1168–72.22. Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, et al. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008; 32:93–103.
Article23. Nanashima A, Sumida Y, Abo T, Tobinaga S, Takeshita H, Hidaka S, et al. A modified grading system for post-hepatectomy metastatic liver cancer originating from colorectal carcinoma. J Surg Oncol. 2008; 98:363–70.
Article24. Schindl M, Wigmore SJ, Currie EJ, Laengle F, Garden OJ. Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg. 2005; 140:183–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Liver Metastases in Colorectal Cancer
- Identifying CT-Based Risk Factors Associated with Synchronous Liver Metastases in Colorectal Cancer
- How to treat liver metastases in colorectal cancer: A review
- Risk Stratification of T1 Colorectal Cancer Metastasis to Lymph Nodes: Current Status and Perspective
- Resection of Colorectal Liver Metastases