Korean J Hepatobiliary Pancreat Surg.
2011 Aug;15(3):146-151. 10.14701/kjhbps.2011.15.3.146.
Scoring of prognostic factors that influence long-term survival in patients with hepatic metastasis of colorectal cancer
- Affiliations
-
- 1Department of Surgery and Research Institute of Clinical Medicine, Chonbuk National University Medical School and Hospital, Jeonju, Korea. hcyu@jbnu.ac.kr
- KMID: 2130985
- DOI: http://doi.org/10.14701/kjhbps.2011.15.3.146
Abstract
- BACKGROUNDS/AIMS
To find independent predictors that affect the survival in patients with hepatic metastasis of colorectal cancer after surgery and to devise a risk scoring system.
METHODS
Among 150 patients who underwent hepatic resection after diagnosis of colorectal cancer with hepatic metastasis between March 1994 and February 2009, we analyzed clinical, pathologic and outcome data retrospectively.
RESULTS
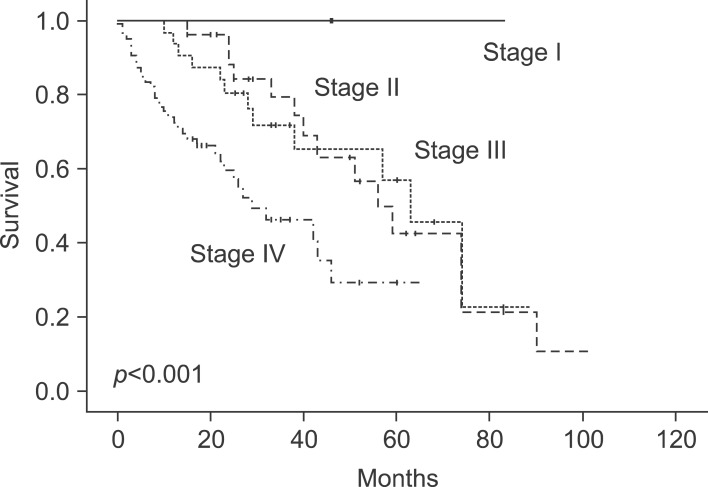
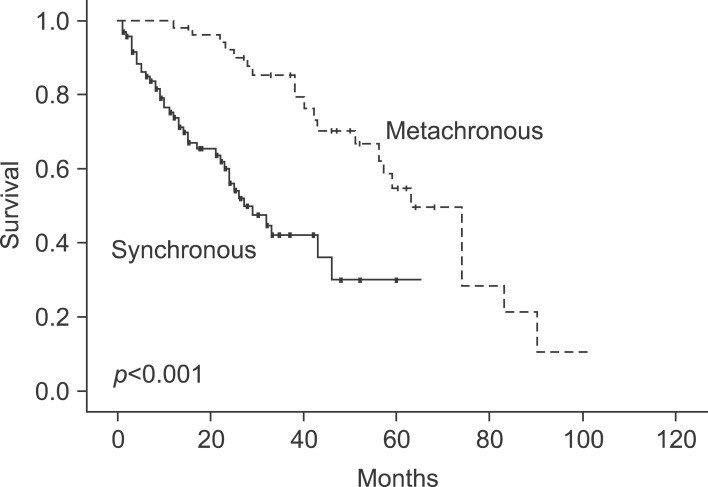
The 1-year survival rate was 83%, and the 5-year survival rate was 35%. Nine factors were found to be independent predictors of adverse outcome by univariate analysis: stage of primary tumor, CA19-9 >36 U/ml, extrahepatic disease, distribution of the hepatic tumor, number of hepatic tumors >3, largest hepatic tumor >5 cm, total size >10 cm, CEA >10 ng/ml, and metachronous cancer. The last two of these criteria were also significant risk factors on multivariate analysis. When these criteria were used as a risk scoring system, assigning one point for each criterion and dividing the cases into A, B and C groups, the total score was highly predictive of outcomes (p<0.001). No patients in group C (6 to 9 points) were long-term survivors.
CONCLUSIONS
Long-term outcome can be predicted from nine criteria that are readily available for all patients. Patients meeting up to two criteria (group A) are more likely to have a favorable outcome compared to the three or over (groups B and C). This scoring system may offer an easy, rapid, and reliable prognostic indicator of survival outcome after hepatic resection in patients with hepatic metastasis from colorectal cancer.
MeSH Terms
Figure
Reference
-
1. Lee WK, Kim SB, Cho EH, Hwang DY, Moon SM. Outcomes of a hepatic resection for colorectal-carcinoma liver metastases. J Korean Soc Coloproctol. 2010; 26:204–210.
Article2. Choi PW, Kim HC, Jung SH, et al. Outcomes after a hepatic resection for multiple hepatic metastases from colorectal cancer. J Korean Soc Coloproctol. 2008; 24:100–106.
Article3. Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990; 77:1241–1246. PMID: 2253003.
Article4. Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg. 1986; 73:732–735. PMID: 3756437.
Article5. Li Destri G, Di Cataldo A, Puleo S. Colorectal cancer follow-up: useful or useless? Surg Oncol. 2006; 15:1–12. PMID: 16891116.
Article6. Nordlinger B, Quilichini MA, Parc R, Hannoun L, Delva E, Huguet C. Hepatic resection for colorectal liver metastases. Influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg. 1987; 205:256–263. PMID: 3827361.7. Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer--competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol. 1990; 16:360–365. PMID: 2379594.8. Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991; 110:13–29. PMID: 1866690.9. Iwatsuki S, Shaw BW Jr, Starzl TE. Experience with 150 liver resections. Ann Surg. 1983; 197:247–253. PMID: 6830332.
Article10. Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002; 235:759–766. PMID: 12035031.
Article11. Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004; 239:818–825. PMID: 15166961.
Article12. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230:309–318. PMID: 10493478.13. Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006; 13:1261–1268. PMID: 16947009.
Article14. Fahy BN, D'Angelica M, DeMatteo RP, et al. Synchronous hepatic metastases from colon cancer: changing treatment strategies and results of surgical intervention. Ann Surg Oncol. 2009; 16:361–370. PMID: 19050976.
Article15. Ruiz-casado A, Pereira F. Liver metastases of colon cancer: New therapeutic approaches, Neoadjuvant chemotherapy. Oncologia. 2006; 29:3–15.
Article16. Silen W. Hepatic resection for metastases from colorectal carcinoma is of dubious value. Arch Surg. 1989; 124:1021–1022. PMID: 2673139.
Article17. Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995; 19:59–71. PMID: 7740812.
Article18. Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994; 116:703–710. PMID: 7940169.19. Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992; 216:493–504. PMID: 1417198.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic resection margin predicts survival in colorectal cancer with hepatic metastasis
- Prognostic Factors after Hepatic Resection for Metastatic Colorectal Cancer
- Outcomes of a Hepatic Resection for Colorectal-Carcinoma Liver Metastases
- Prognostic Factors Affecting Survival Rate Following Hepatic Resection for Metastatic Colorectal Cancer
- Clinical Applications of Radio-Frequency Ablation in Liver Metastasis of Colorectal Cancer