Hanyang Med Rev.
2015 Nov;35(4):222-228. 10.7599/hmr.2015.35.4.222.
Recent Advances in Skeletal Muscle Stem Cells for Duchenne Muscular Dystrophy Treatment
- Affiliations
-
- 1Department of Surgery, Hanyang University College of Medicine, Seoul, Korea. jmj1103@hanyang.ac.kr
- KMID: 2129475
- DOI: http://doi.org/10.7599/hmr.2015.35.4.222
Abstract
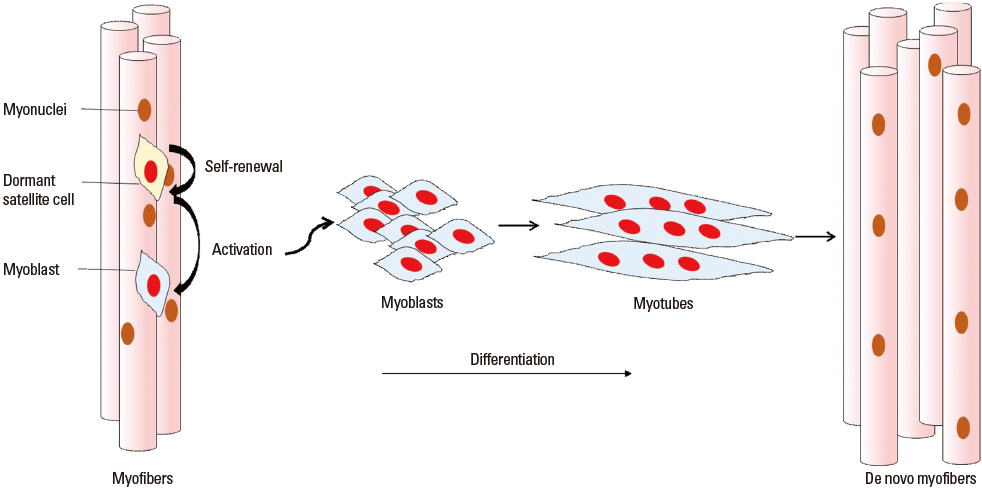
- Muscle stem cells, which are known as satellite cells have heterogeneous components of committed myogenic progenitors, non-committed satellite cells, and mesenchymal stem cells. This distinguishing organization of self-renewal and differentiation capacities encourages the remarkable regenerative ability of skeletal muscles. Lately it has been proved that the satellite cell is the derivation of muscle regeneration and with the self-renew function, it roles as a true muscle stem cell. Therefore, stem cell therapy using satellite cells is considered to be ideal therapy for muscular dystrophies, which is deficient in specific muscle protein and causes muscle degeneration. Especially, Duchenne Muscular Dystrophy (DMD), which is caused by mutations at the dystrophin gene, has been targeted by much research. In this article the satellite cell characteristics, regulation of cell function, and stem cell therapy for DMD and the present progressive clinical trials will be reviewed.
MeSH Terms
Figure
Cited by 1 articles
-
New Horizons in Stem Cell Research
Dongho Choi
Hanyang Med Rev. 2015;35(4):187-189. doi: 10.7599/hmr.2015.35.4.187.
Reference
-
1. Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat Rec. 1977; 188:201–217.
Article2. Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005; 122:289–301.
Article3. Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007; 129:999–1010.
Article4. Emery AE. The muscular dystrophies. Lancet. 2002; 359:687–695.
Article5. Cossu G, Sampaolesi M. New therapies for duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007; 13:520–526.
Article6. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961; 9:493–495.
Article7. Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008; 14:82–91.
Article8. Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat Rec. 1975; 182:215–235.
Article9. Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004; 84:209–238.
Article10. Nagata Y, Partridge TA, Matsuda R, Zammit PS. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol. 2006; 174:245–253.
Article11. Wozniak AC, Anderson JE. Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn. 2007; 236:240–250.
Article12. Pisconti A, Brunelli S, Di Padova M, De Palma C, Deponti D, Baesso S, et al. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol. 2006; 172:233–244.
Article13. Jones NC, Tyner KJ, Nibarger L, Stanley HM, Cornelison DD, Fedorov YV, et al. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol. 2005; 169:105–116.
Article14. Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971; 170:421–435.
Article15. Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004; 116:769–778.16. Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008; 2:22–31.
Article17. Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007; 18:1397–1409.
Article18. Quarta M, Rando TA. Mimicking the niche: cytokines expand muscle stem cells. Cell Res. 2015; 25:761–762.
Article19. Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012; 10:504–514.
Article20. Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012; 30:232–242.
Article21. Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002; 3:397–409.
Article22. Abou-Khalil R, Brack AS. Muscle stem cells and reversible quiescence: the role of sprouty. Cell Cycle. 2010; 9:2575–2580.
Article23. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003; 162:1135–1147.
Article24. Kitamoto T, Hanaoka K. Notch3 null mutation in mice causes muscle hyperplasia by repetitive muscle regeneration. Stem Cells. 2010; 28:2205–2216.
Article25. Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, et al. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010; 6:117–129.
Article26. Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse numb and numbl in maintaining progenitor cells during cortical neurogenesis. Nat Neurosci. 2004; 7:803–811.
Article27. Church JC. Satellite cells and myogenesis; a study in the fruit-bat web. J Anat. 1969; 105:419–438.28. Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 2007; 3:226–237.
Article29. McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, et al. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008; 10:77–84.
Article30. Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000; 102:777–786.
Article31. Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000; 151:1221–1234.
Article32. Collins CA, Gnocchi VF, White RB, Boldrin L, Perez-Ruiz A, Relaix F, et al. Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS One. 2009; 4:e4475.
Article33. Meech R, Gonzalez KN, Barro M, Gromova A, Zhuang L, Hulin JA, et al. Barx2 is expressed in satellite cells and is required for normal muscle growth and regeneration. Stem Cells. 2012; 30:253–265.
Article34. Hollnagel A, Grund C, Franke WW, Arnold HH. The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol Cell Biol. 2002; 22:4760–4770.
Article35. Wozniak AC, Pilipowicz O, Yablonka-Reuveni Z, Greenway S, Craven S, Scott E, et al. C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem. 2003; 51:1437–1445.
Article36. Ozeki N, Lim M, Yao CC, Tolar M, Kramer RH. alpha7 integrin expressing human fetal myogenic progenitors have stem cell-like properties and are capable of osteogenic differentiation. Exp Cell Res. 2006; 312:4162–4180.
Article37. Ieronimakis N, Balasundaram G, Rainey S, Srirangam K, Yablonka-Reuveni Z, Reyes M. Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PLoS One. 2010; 5:e10920.
Article38. Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003; 21:363–371.
Article39. Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001; 239:79–94.
Article40. Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One. 2009; 4:e5205.
Article41. Yamaguchi M, Ogawa R, Watanabe Y, Uezumi A, Miyagoe-Suzuki Y, Tsujikawa K, et al. Calcitonin receptor and Odz4 are differently expressed in Pax7-positive cells during skeletal muscle regeneration. J Mol Histol. 2012; 43:581–587.
Article42. Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006; 20:486–500.
Article43. Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000; 10:113–120.
Article44. Manzur AY, Kinali M, Muntoni F. Update on the management of duchenne muscular dystrophy. Arch Dis Child. 2008; 93:986–990.
Article45. Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007; 15:867–877.
Article46. Guerette B, Asselin I, Skuk D, Entman M, Tremblay JP. Control of inflammatory damage by anti-LFA-1: increase success of myoblast transplantation. Cell Transplant. 1997; 6:101–107.
Article47. Skuk D, Roy B, Goulet M, Tremblay JP. Successful myoblast transplantation in primates depends on appropriate cell delivery and induction of regeneration in the host muscle. Exp Neurol. 1999; 155:22–30.
Article48. Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989; 337:176–179.
Article49. Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005; 309:314–317.
Article50. Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006; 444:574–579.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Gastric Dilatation and Calculous Cholecystitis in Duchenne's Muscular Dystrophy
- A clinical study on Duchenne muscular dystrophy
- Transplantation of Differentiated Tonsil-Derived Mesenchymal Stem Cells Ameliorates Murine Duchenne Muscular Dystrophy via Autophagy Activation
- Duchenne Type Muscular Dystrophy: Report of 8 Cases
- Gene Therapy of Inherited Muscle Diseases