Clin Exp Otorhinolaryngol.
2015 Sep;8(3):189-193. 10.3342/ceo.2015.8.3.189.
Factors Affecting the Variation of Maximum Speech Intelligibility in Patients With Sensorineural Hearing Loss Other Than Apparent Retrocochlear Lesions
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Tohoku University Graduate School of Medicine, Sendai, Japan. kawase@orl.med.tohoku.ac.jp
- 2Department of Audiology, Tohoku University Graduate School of Medicine, Sendai, Japan.
- 3Laboratory of Rehabilitative Auditory Science, Tohoku University Graduate School of Biomedical Engineering, Sendai, Japan.
- KMID: 2117509
- DOI: http://doi.org/10.3342/ceo.2015.8.3.189
Abstract
OBJECTIVES
To examine the relationship between speech intelligibilities among the similar level of hearing loss and threshold elevation of the auditory brainstem response (ABR).
METHODS
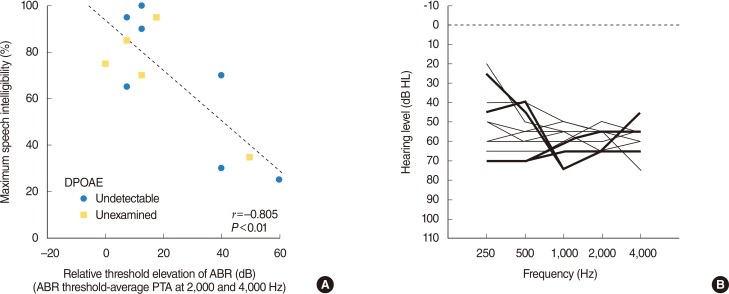
The relationship between maximum speech intelligibilities among similar levels of hearing loss and relative threshold elevation of the click-evoked ABR (ABR threshold - pure tone average at 2,000 and 4,000 Hz) was retrospectively reviewed in patients with sensorineural hearing loss (SNHL) other than apparent retrocochlear lesions as auditory neuropathy, vestibular schwannoma and the other brain lesions.
RESULTS
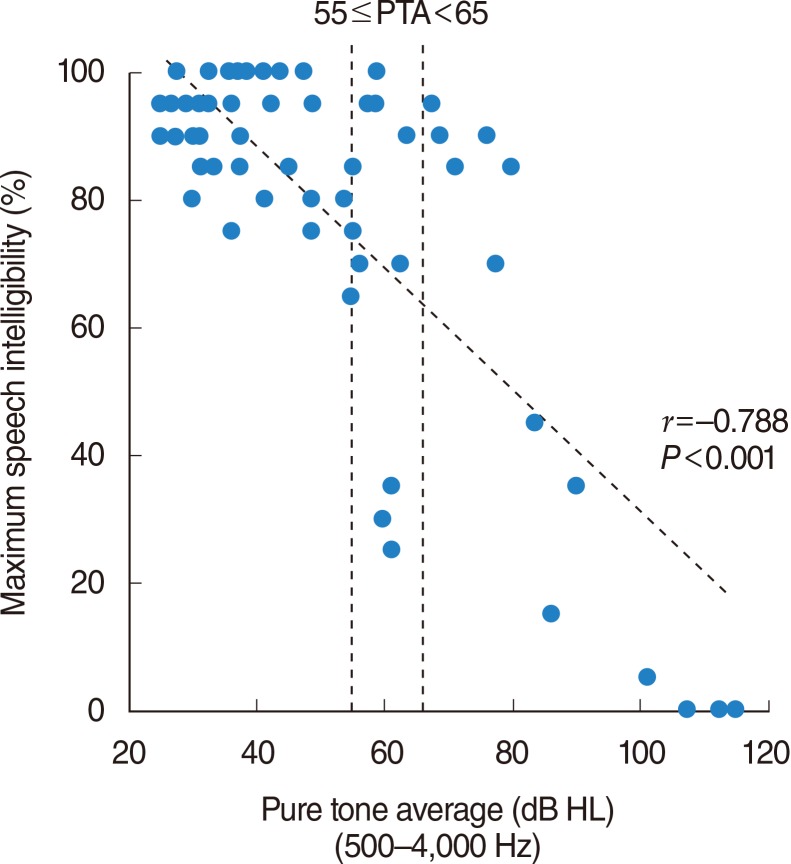
Comparison of the speech intelligibilities in subjects with similar levels of hearing loss found that the variation in maximum speech intelligibility was significantly correlated with the threshold elevation of the ABR.
CONCLUSION
The present results appear to support the idea that variation in maximum speech intelligibility in patients with similar levels of SNHL may be related to the different degree of dysfunctions of the inner hair cells and/or cochlear nerves in addition to those of outer hair cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Fletcher H. A method of calculating hearing loss for speech from an audiogram. Acta Otolaryngol Suppl. 1950; 90:26–37. PMID: 14818775.
Article2. Carhart R. Observations on relations between thresholds for pure tones and for speech. J Speech Hear Disord. 1971; 11. 36(4):476–483. PMID: 5125799.
Article3. Johnson EW. Auditory findings in 200 cases of acoustic neuromas. Arch Otolaryngol. 1968; 12. 88(6):598–604. PMID: 5724832.
Article4. Penrod JP. Speech threshold and word recognition/discrimination testing. In : Katz J, Gabbay WL, editors. Handbook of clinical audiology. 4th ed. Baltimore: Williams & Wilkins;1994. p. 147–164.5. Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996; 6. 119(Pt 3):741–753. PMID: 8673487.
Article6. Kaga K, Nakamura M, Shinogami M, Tsuzuku T, Yamada K, Shindo M. Auditory nerve disease of both ears revealed by auditory brainstem responses, electrocochleography and otoacoustic emissions. Scand Audiol. 1996; 25(4):233–238. PMID: 8975994.
Article7. Amatuzzi M, Liberman MC, Northrop C. Selective inner hair cell loss in prematurity: a temporal bone study of infants from a neonatal intensive care unit. J Assoc Res Otolaryngol. 2011; 10. 12(5):595–604. PMID: 21674215.
Article8. Manchaiah VK, Zhao F, Danesh AA, Duprey R. The genetic basis of auditory neuropathy spectrum disorder (ANSD). Int J Pediatr Otorhinolaryngol. 2011; 2. 75(2):151–158. PMID: 21176974.
Article9. Santarelli R, Del Castillo I, Rodríguez-Ballesteros M, Scimemi P, Cama E, Arslan E, et al. Abnormal cochlear potentials from deaf patients with mutations in the otoferlin gene. J Assoc Res Otolaryngol. 2009; 12. 10(4):545–556. PMID: 19636622.
Article10. Liberman MC, Tartaglini E, Fleming JC, Neufeld EJ. Deletion of SLC19A2, the high affinity thiamine transporter, causes selective inner hair cell loss and an auditory neuropathy phenotype. J Assoc Res Otolaryngol. 2006; 9. 7(3):211–217. PMID: 16642288.
Article11. Ngo RY, Tan HK, Balakrishnan A, Lim SB, Lazaroo DT. Auditory neuropathy/auditory dys-synchrony detected by universal newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2006; 7. 70(7):1299–1306. PMID: 16417926.
Article12. Kaga K, Iwasaki S, Tamura A, Suzuki J, Haebara H. Temporal bone pathology of acoustic neuroma correlating with presence of electrocochleography and absence of auditory brainstem response. J Laryngol Otol. 1997; 10. 111(10):967–972. PMID: 9425489.
Article13. Kveton JF. Delayed spontaneous return of hearing after acoustic tumor surgery: evidence for cochlear nerve conduction block. Laryngoscope. 1990; 5. 100(5):473–476. PMID: 2329903.14. Michalewski HJ, Starr A, Nguyen TT, Kong YY, Zeng FG. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin Neurophysiol. 2005; 3. 116(3):669–680. PMID: 15721081.
Article15. Roberson JB Jr, Jackson LE, McAuley JR. Acoustic neuroma surgery: absent auditory brainstem response does not contraindicate attempted hearing preservation. Laryngoscope. 1999; 6. 109(6):904–910. PMID: 10369280.
Article16. Satya-Murti S, Wolpaw JR, Cacace AT, Schaffer CA. Late auditory evoked potentials can occur without brain stem potentials. Electroencephalogr Clin Neurophysiol. 1983; 10. 56(4):304–308. PMID: 6193943.
Article17. Starr A, McPherson D, Patterson J, Don M, Luxford W, Shannon R, et al. Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain. 1991; 6. 114(Pt 3):1157–1180. PMID: 2065245.
Article18. Takata Y, Kawase T, Nakasato N, Kanno A, Kobayashi T. Auditory evoked magnetic fields in patients with absent brainstem responses due to auditory neuropathy with optic atrophy. Clin Neurophysiol. 2012; 5. 123(5):985–992. PMID: 22119798.
Article19. Baldwin M, Watkin P. Predicting the degree of hearing loss using click auditory brainstem response in babies referred from newborn hearing screening. Ear Hear. 2013; May-Jun. 34(3):361–369. PMID: 23340456.
Article20. van der Drift JF, Brocaar MP, van Zanten GA. The relation between the pure-tone audiogram and the click auditory brainstem response threshold in cochlear hearing loss. Audiology. 1987; 26(1):1–10. PMID: 3593096.21. Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993; 1. 102(1 Pt 2):1–16. PMID: 8420477.
Article22. Nadol JB Jr. Disorder of aging. In : Merchant SN, Nadol JB, editors. Schuknecht's pathology of the ear. 3rd ed. Shelton (CT): PMPH-USA;2010. p. 431–476.23. Adams JC, Merchant SN. Disorders of intoxication. In : Merchant SN, Nadol JB, editors. Schuknecht's pathology of the ear. 3rd ed. Shelton (CT): PMPH-USA;2010. p. 353–380.24. Merchant SN, Liberman MC. Trauma. In : Merchant SN, Nadol JB, editors. Schuknecht's pathology of the ear. 3rd ed. Shelton (CT): PMPH-USA;2010. p. 381–412.25. Kohonen A. Effect of some ototoxic drugs upon the pattern and innervation of cochlear sensory cells in the guinea pig. Acta Otolaryngol Suppl. 1965; (Suppl 208):1–70. PMID: 4159018.26. Ylikoski J. Correlative studies on the cochlear pathology and hearing loss in guinea-pigs after intoxication with ototoxic antibiotics. Acta Otolaryngol Suppl. 1974; 326:1–62. PMID: 4534029.27. Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken). 012; 11. 295(11):1837–1850. PMID: 23045231.
Article28. Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: a review and tutorial. J Acoust Soc Am. 1985; 9. 78(3):833–860. PMID: 4040933.
Article29. Merchant SN. Degeneration of auditory and vestibular end organs. In : Merchant SN, Nadol JB, editors. Schuknecht's pathology of the ear. 3rd ed. Shelton (CT): PMPH-USA;2010. p. 631–664.30. Kiang NY, Liberman MC, Sewell WF, Guinan JJ. Single unit clues to cochlear mechanisms. Hear Res. 1986; 22(1-3):171–182. PMID: 3733538.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improvement of Speech Intelligibility in Patients with Sensorineural Hearing Loss Using Noise Reduction Algorithms
- Speech Intelligibility in Persian Hearing Impaired Children with Cochlear Implants and Hearing Aids
- A Case of Acute Bilateral Retrocochlear Hearing Loss as an Initial Symptom of Unilateral Thalamic Hemorrhage
- A Personal Sound Amplification Product Compared to aBasic Hearing Aid for Speech Intelligibility in Adults withMild-to-Moderate Sensorineural Hearing Loss
- Efficacy of Audiologic Tests in the Differential Diagnosis of Cochlear and Retrocochlear Hearing Loss