J Korean Soc Radiol.
2012 Sep;67(3):177-186. 10.3348/jksr.2012.67.3.177.
The Predictability for the Prognosis of Breast Cancer Using the Apparent Diffusion Coefficient Value of Diffusion-Weighted 3 T MRI and the Standardized Uptake Value of Positron Emission Tomography/CT: Assessment of Prognostic Factor
- Affiliations
-
- 1Department of Radiology, Konyang University College of Medicine, Daejeon, Korea. lizkim1@hanmail.net
- 2Department of Pathology, Konyang University College of Medicine, Daejeon, Korea.
- 3Department of Nuclear Medicine, Konyang University College of Medicine, Daejeon, Korea.
- 4Department of Preventive Medicine, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2097969
- DOI: http://doi.org/10.3348/jksr.2012.67.3.177
Abstract
- PURPOSE
To correlate the apparent diffusion coefficient (ADC) value and peak standardized uptake value (pSUV) with histologic grade and clinical prognostic factors of breast ductal carcinoma.
MATERIALS AND METHODS
Fifty breast cancers of 49 patients (age range: 37-83 years, mean: 53 years) were studied retrospectively. The breast cancers included 4 ductal carcinoma in situ (DCIS) and 46 invasive ductal carcinomas (IDC). The relationships for both pSUV and ADC values with clinicopathological prognostic factors (age, tumor size, histologic grade, nodal metastasis, hormone receptor and HER-2 neu status) were statistically evaluated.
RESULTS
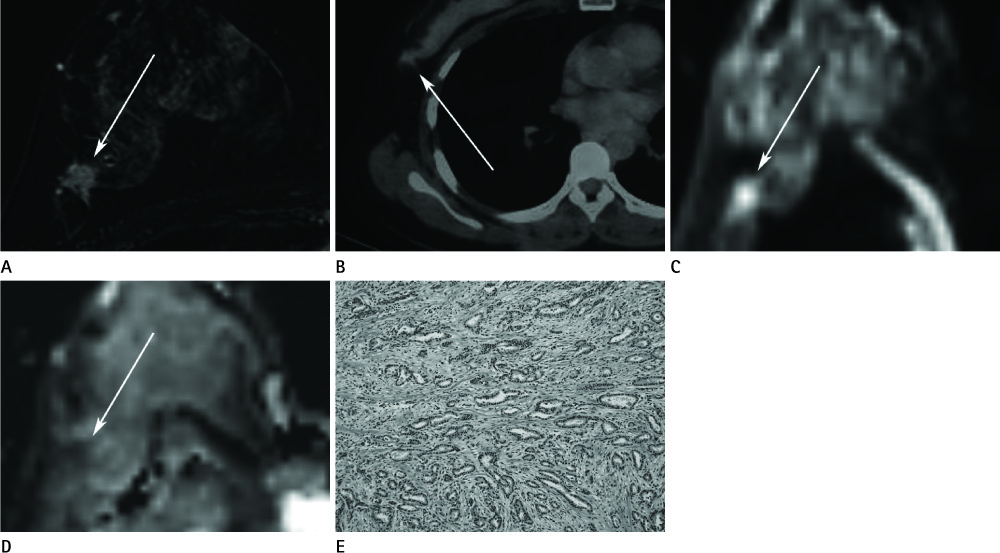
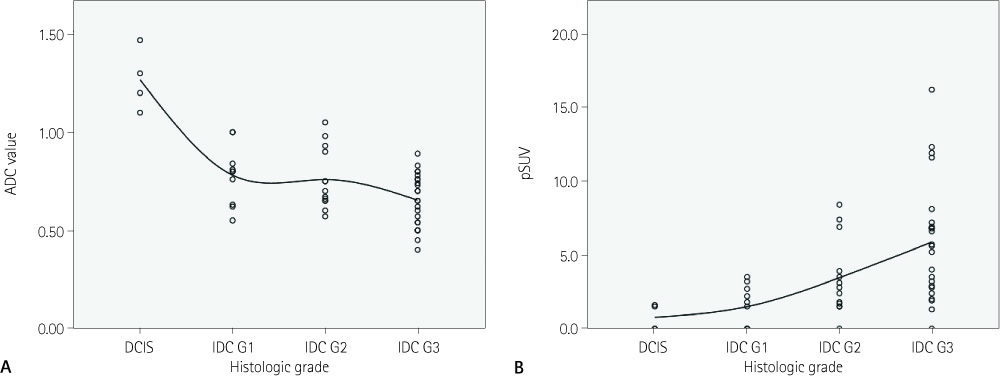
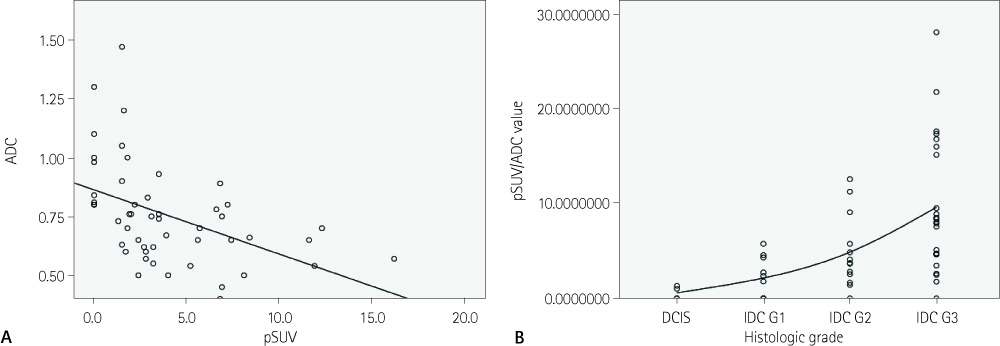
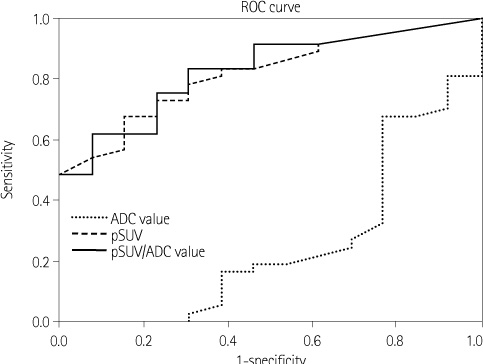
The histologic type of ductal carcinoma include DCIS (n = 4) and IDC (n = 46, grade 1 = 10, grade 2 = 13, and grade 3 = 23). pSUV was associated with histologic grade and tumor size and the ADC value was associated with histologic grade (p < 0.05). As the histologic grade becomes higher, the ADC values decrease, while pSUV and pSUV/ADC increase (p < 0.05). The characterization accuracy of pSUV/ADC (90.2%) was higher than pSUV (86.7%) and ADC values (25.4%) alone for the diagnosis of breast cancer (p < 0.05).
CONCLUSION
pSUV and ADC values correlated with histologic grade, and tumor size. The pSUV/ADC value had a high accuracy for the diagnosis of breast cancer. Therefore, pSUV and ADC values provided additional information for predicting histologic grade and prognosis of breast cancer.
MeSH Terms
Figure
Reference
-
1. Orel SG. High-resolution MR imaging for the detection, diagnosis, and staging of breast cancer. Radiographics. 1998; 18:903–912.2. Ikeda DM, Baker DR, Daniel BL. Magnetic resonance imaging of breast cancer: clinical indications and breast MRI reporting system. J Magn Reson Imaging. 2000; 12:975–983.3. Bluemke DA, Gatsonis CA, Chen MH, DeAngelis GA, DeBruhl N, Harms S, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004; 292:2735–2742.4. Siegmann KC, Müller-Schimpfle M, Schick F, Remy CT, Fersis N, Ruck P, et al. MR imaging-detected breast lesions: histopathologic correlation of lesion characteristics and signal intensity data. AJR Am J Roentgenol. 2002; 178:1403–1409.5. Bogner W, Gruber S, Pinker K, Grabner G, Stadlbauer A, Weber M, et al. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology. 2009; 253:341–351.6. Belli P, Costantini M, Bufi E, Magistrelli A, La Torre G, Bonomo L. Diffusion-weighted imaging in breast lesion evaluation. Radiol Med. 2010; 115:51–69.7. Park MJ, Cha ES, Kang BJ, Ihn YK, Baik JH. The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol. 2007; 8:390–396.8. Ahn HS, Chang YW, Choi KH, Kim HJ, Hong SS, Hwang JH, et al. Usefulness of diffusion-weighted MR imaging for breast lesions: comparing the apparent diffusion coefficient (ADC) values and the pathologic results. J Korean Soc Radiol. 2011; 64:375–381.9. Marini C, Iacconi C, Giannelli M, Cilotti A, Moretti M, Bartolozzi C. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol. 2007; 17:2646–2655.10. Heusner TA, Kuemmel S, Koeninger A, Hamami ME, Hahn S, Quinsten A, et al. Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging. Eur J Nucl Med Mol Imaging. 2010; 37:1077–1086.11. Costantini M, Belli P, Rinaldi P, Bufi E, Giardina G, Franceschini G, et al. Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clin Radiol. 2010; 65:1005–1012.12. Bae SY, Lee EH, Park JM, Kwak JJ. 18F-fluorodeoxyglucose positron emission tomography/CT scan findings for ductal carcinomas of breast: association of standardized uptake value and histological findings. J Korean Soc Radiol. 2012; 66:169–175.13. Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009; 50:1820–1827.14. Crowe JP Jr, Adler LP, Shenk RR, Sunshine J. Positron emission tomography and breast masses: comparison with clinical, mammographic, and pathological findings. Ann Surg Oncol. 1994; 1:132–140.15. Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer. 1998; 82:2227–2234.16. Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and breast cancer imaging. Radiographics. 2007; 27:Suppl 1. S215–S229.17. Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko ES, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008; 9:10–18.18. Jeong SH, Lee EH, Park JM, Lee HK, Yi BH, Choi N. Factors affecting 18F-fluorodeoxyglucose (FDG) uptake in breast cancer. J Korean Soc Radiol. 2010; 63:287–292.19. Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010; 37:2011–2020.20. Vargas HA, Akin O, Franiel T, Mazaheri Y, Zheng J, Moskowitz C, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011; 259:775–784.21. Park SH, Moon WK, Cho N, Chang JM, Im SA, Park IA, et al. Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol. 2012; 22:18–25.22. Riegger C, Herrmann J, Nagarajah J, Hecktor J, Kuemmel S, Otterbach F, et al. Whole-body FDG PET/CT is more accurate than conventional imaging for staging primary breast cancer patients. Eur J Nucl Med Mol Imaging. 2012; 39:852–863.23. Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006; 98:267–274.24. Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010; 23:619–623.25. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.26. Elston CW. Classification and grading of invasive breast carcinoma. Verh Dtsch Ges Pathol. 2005; 89:35–44.27. Kuhl CK. MRI of breast tumors. Eur Radiol. 2000; 10:46–58.28. Bartella L, Smith CS, Dershaw DD, Liberman L. Imaging breast cancer. Radiol Clin North Am. 2007; 45:45–67.29. Buck A, Schirrmeister H, Kühn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002; 29:1317–1323.30. Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008; 38:250–258.31. Fueger BJ, Weber WA, Quon A, Crawford TL, Allen-Auerbach MS, Halpern BS, et al. Performance of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography and integrated PET/CT in restaged breast cancer patients. Mol Imaging Biol. 2005; 7:369–376.32. Tatsumi M, Cohade C, Mourtzikos KA, Fishman EK, Wahl RL. Initial experience with FDG-PET/CT in the evaluation of breast cancer. Eur J Nucl Med Mol Imaging. 2006; 33:254–262.33. Crippa F, Seregni E, Agresti R, Chiesa C, Pascali C, Bogni A, et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998; 25:1429–1434.34. Hatakenaka M, Soeda H, Yabuuchi H, Matsuo Y, Kamitani T, Oda Y, et al. Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci. 2008; 7:23–29.35. Kawashima M, Tamaki Y, Nonaka T, Higuchi K, Kimura M, Koida T, et al. MR imaging of mucinous carcinoma of the breast. AJR Am J Roentgenol. 2002; 179:179–183.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Apparent Diffusion Coefficient Value of Diffusion-Weighted Imaging and Peak Standardized Uptake Values of Positron Emission Tomography-CT for Predicting Prognostic Factors of Breast Cancer
- Correlation of Prognostic Factors of Invasive Lobular Carcinoma with ADC Value of DWI and SUVMax of FDG-PET
- Diffusion-Weighted MRI for the Initial Viability Evaluation of Parasites in Hepatic Alveolar Echinococcosis: Comparison with Positron Emission Tomography
- Diffusion-Weighted MRI for the Assessment of Molecular Prognostic Biomarkers in Breast Cancer
- Reversal of a Large Ischemic Lesion with Low Apparent Diffusion Coefficient Value by Rapid Spontaneous Recanalization