J Korean Soc Radiol.
2010 Nov;63(5):463-469. 10.3348/jksr.2010.63.5.463.
Islet Cell Tumors of the Pancreas: A Variety of MultiphaseDynamic Imaging Findings with Pathologic Correlations Focusing on Nonfunctioning Tumors and Insulinomas
- Affiliations
-
- 1Department of Diagnostic Radiology, Gangnam Severance Hospital,Yonsei University College of Medicine, Korea. yjsrad97@yuhs.ac
- 2Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Korea.
- KMID: 2097919
- DOI: http://doi.org/10.3348/jksr.2010.63.5.463
Abstract
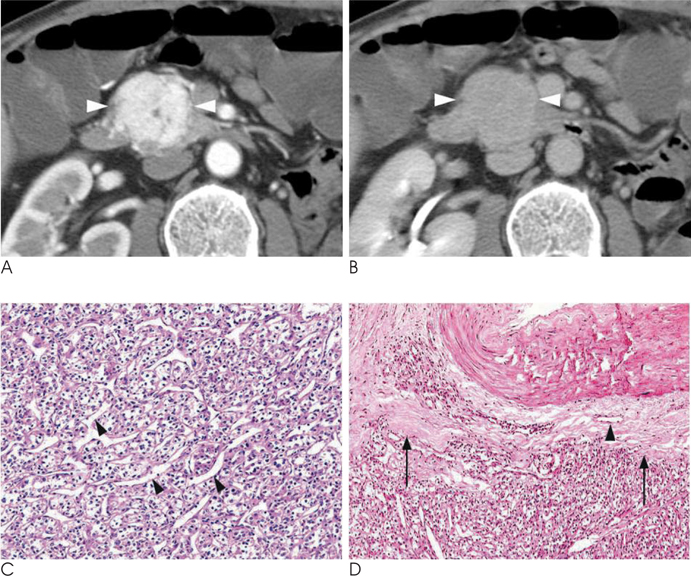
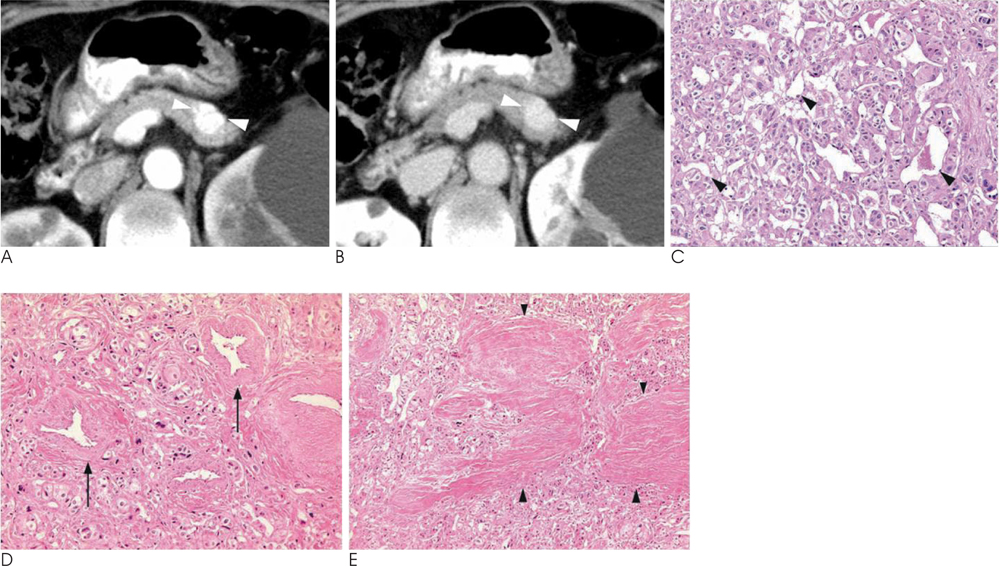
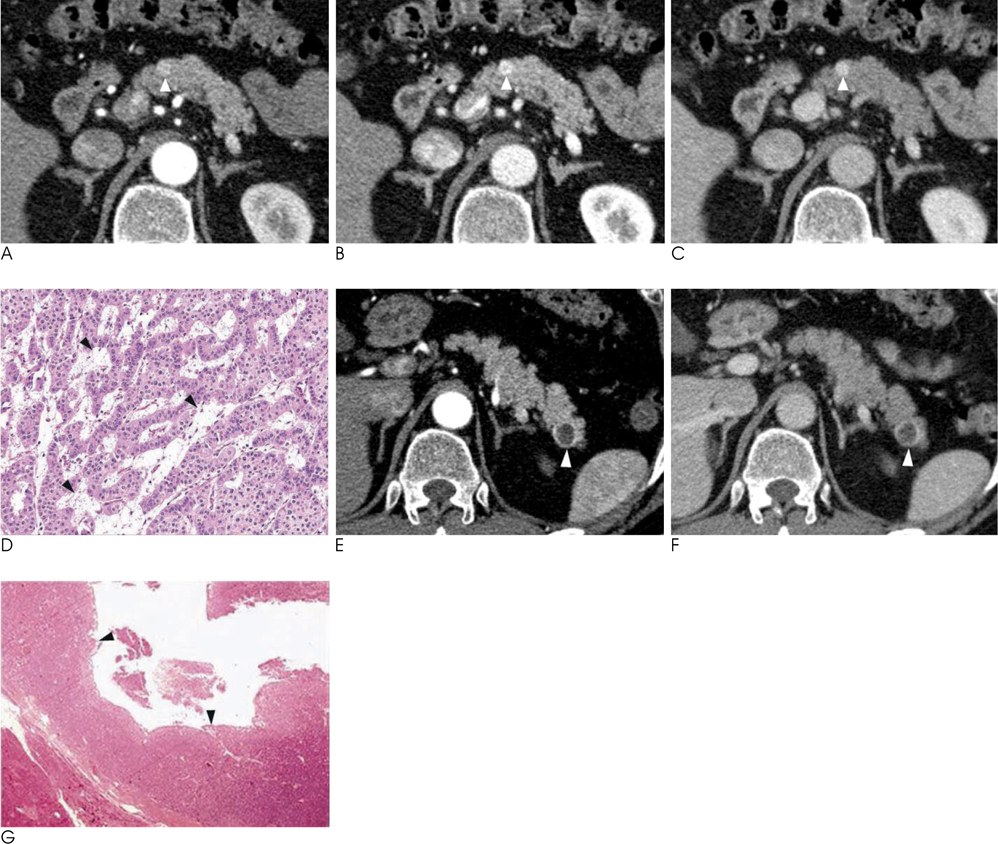
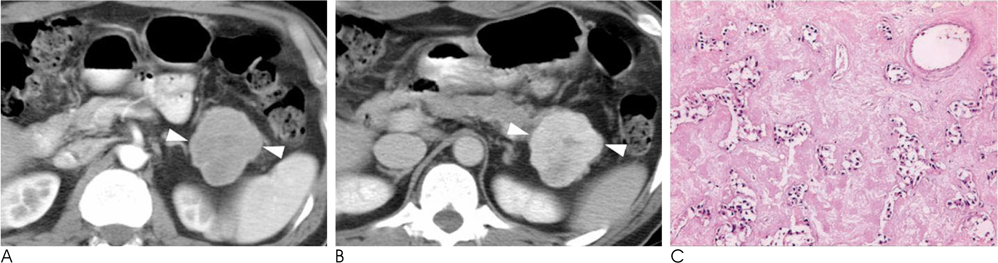
- Islet cell tumors (ICTs) are rare pancreatic neoplasms of neuroendocrine origin, posing a diagnostic challenge to radiologists. We illustrated a spectrum of features of pancreatic ICTs that could be found on multiphase dynamic CT or MRI, and elucidated the histopathologic characteristics by determining the contrast enhancement pattern of the lesions. Various enhancement patterns were dependant on the internal composition of the tumor, that is, the proportion of vascular densities for early enhancement and non-hypervascular interstitial tissue for late enhancement regardless of the size or functional behavior. This knowledge of the imaging-pathologic spectrum of ICTs could be helpful for the proper differential diagnosis from other pancreatic tumors.
MeSH Terms
Figure
Reference
-
1. Eriguchi N, Aoyagi S, Hara M, Fukuda S, Tanaka E, Hashimoto M. Nonfunctioning islet cell carcinoma of the pancreas: an evaluation of seven patients who underwent resection followed by long-term survival. Surg Today. 1999; 29:233–237.2. Procacci C, Carbognin G, Accordini S, Biasiutti C, Bicego E, Romano L, et al. Nonfunctioning endocrine tumors of the pancreas: possibilities of spiral CT characterization. Eur Radiol. 2001; 11:1175–1183.3. Sheth S, Hruban RK, Fishman EK. Helical CT of islet cell tumors of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2002; 179:725–730.4. Rodallec M, Vilgrain V, Couvelard A, Rufat P, O'Toole D, Barrau V, et al. Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology. 2006; 6:77–85.5. McAuley G, Delaney H, Colville J, Lyburn L, Worsley D, Govender P, et al. Multimodality preoperative imaging of pancreatic insulinomas. Clin Radiol. 2005; 60:1039–1050.6. d'Assignies G, Couvelard A, Bahrami S, Vullierme MP, Hammel P, Hentic O, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009; 250:407–416.7. Ichikawa T, Peterson MS, Federle MP, Baron RL, Haradome H, Kawamori Y, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000; 216:163–171.8. Iglesias A, Arias M, Casal M, Páramo C, Fiaño C, Brasa J. Unusual presentation of a pancreatic insulinoma in helical CT and dynamic contrast-enhanced MR imaging: case report. Eur Radiol. 2001; 11:926–930.9. Itai Y, Ohtomo K, Kokubo T, Yamauchi T, Minami M, Yashiro N, et al. CT of hepatic masses: significance of prolonged and delayed enhancement. AJR Am J Roentgenol. 1986; 146:729–733.10. Park MS, Kim KH, Lim JS, Lee JH, Kim JH, Kim SY, et al. Unusual cystic neoplasms in the pancreas: radiologic-pathologic correlation. J Comput Assist Tomogr. 2005; 29:610–616.