J Korean Soc Spine Surg.
2008 Jun;15(2):115-131. 10.4184/jkss.2008.15.2.115.
Current Status of Lumbar Total Disc Replacement (TDR)
- Affiliations
-
- 1Department of Orthopedic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. csl3503@skku.edu
- KMID: 2097842
- DOI: http://doi.org/10.4184/jkss.2008.15.2.115
Abstract
- Fusion surgery remains the gold standard for surgical treatment of lumbar degenerative disc disease (DDD). However, fusion surgery has many intrinsic problems related to altered biomechanics and balance. Total disc replacement (TDR) has received increasing attention over the last several years because of its capacity for both functional and symptomatic improvement and its avoidance of problems intrinsic to fusion surgery. Artificial disc replacement is not a new concept, the first attempts having been undertaken in the early 1950s. However, during the past 15 years, considerable advances have been made, with clinical success noted in several prospective randomized studies and mid-long term retrospective studies. Proper patient selection and surgical technique are key factors in achieving a successful outcome. TDR plays a limited role and has limited indications for replacing fusion surgery in patients with lumbar DDD. The main goal of TDR is restoration of normal intervertebral disc function. Varying degrees of motion can be restored through TDR; however, the pattern of motion and center of rotation are not physiologic. In spite of some favorable reports, many TDR-related problems remain to be solved. Successful disc function is measured not only in terms of quantity of motion, but also in terms of quality of motion and shock energy absorption capacity. For successful repair to be declared, facet unloading should be achieved, and fatigue strength should be improved. New procedures should be characterized by a reduction in the technical problems of implantation and retrieval. We expect that the next generation of TDR will overcome the limitations of first generation TDR. This therapeutic modality shows much promise for the treatment of lumbar DDD.
Keyword
MeSH Terms
Figure
Reference
-
1). Errico TJ. Lumbar disc arthroplasty. Clin Orthop Relat Res. 2005; 435:106–117.
Article2). Fritzell P, Hagg O, Wessberg P, Nordwall A. Chronic low back pain and fusion: A comparison of three surgical techniques: A prospective multicenter randomized study from the Swedish Lumbar spine study group. Spine. 2002; 27:1131–1141.3). Liu J, Ebraheim NA, Haman SP, et al. Effect of the increase in the height of lumbar disc space on facet joint articulation area in sagittal plane. Spine. 2006; 31:198–202.
Article4). Chung SS, Lee CS, Kang CS, Kim SH. The Effect of Lumbar Total Disc Replacement on the Spinopelvic Alignment and Range of Motion of the Lumbar Spine. J Spinal Disord Tech. 2006; 19:307–311.
Article5). Tournier C, Aunoble S, Le Huec JC, et al. Total disc arthroplasty: consequences for sagittal balance and lumbar spine movement. Eur Spine J. 2007; 16:411–421.
Article6). Fernstrom U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand. 1966; 357:154–159.7). Buttner-Janz K. The development of the Artificial Disc Charite SB. Dallas, TX: Hudley & Associates;1992.8). Enker P, Steffee A, McMillin C, et al. Artificial disc replacement. Preliminary report with a 3-year minimum follew-up. Spine. 1993; 18:1061–1070.9). Steffee AD. The Steffee artificial disc. Weinstein JN, editor. Clinical Dfficacy and Outcome in the diagnosis and treatment of low back pain. New York, NY: Raven Press;1992. p. 245–257.10). Marnay T. Lumbar disc replacement. 7-11 years results with Prodisc. Eur Spine J. 2002; 11:19.11). Huang RC, Girardi FP, Cammisa Jr FP, Tropiano P, Marnay T. Long-term flexion-extension range of motion of the prodisc total disc replacement. J Spinal Disord Tech. 2003; 16:435–440.
Article12). Tropiano P, Huang RC, Girardi FP, Cammisa FP Jr, Marnay T. Lumbar total disc replacemtent. Seven to eleven-year follow-up. J Bone Joint Surg Am. 2005; 87:490–496.13). Lee CK, Goel VK. Artificial disc prosthesis: design concepts and criteria. Spine J. 2004; 4:209–218.
Article14). Lemaire JP, Carrier H, Sariali el-H, Skalli W, Lavaste F. Clinical and radiological outcomes with the Charite´ artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005; 18:353–359.15). Cinotti G, David T, Postacchini F. Results of disc Prosthesis after a Minimum follow-up period of 2 years. Spine. 1996; 21:995–1000.
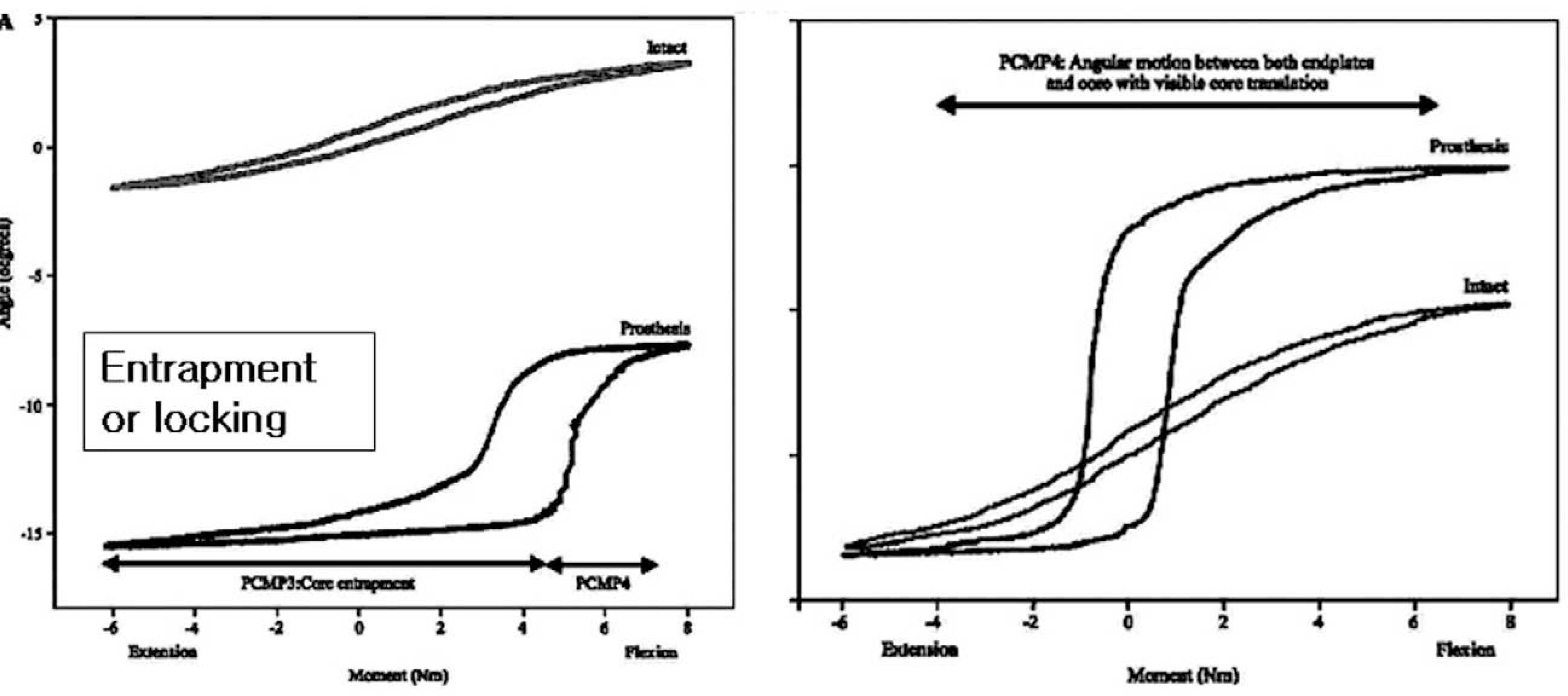
Article16). Rousseau MA, Bradford DS, Bertagnoli R, Hu SS, Lotz JC. Disc arthroplasty design influences intervertebral kinematics and facet forces. Spine J. 2006; 6:258–266.
Article17). O'Leary P, Nicolakis M, Lorenz MA, et al. Response of Charite´ total disc replacement under physiologic loads: prosthesis component motion patterns. Spine J. 2005; 5:590–599.18). Auerbach JD, Willis BP, McIntosh TC, Balderston RA. Evaluation of spinal kinematics following lumbar total disc replacement and circumferential fusion using in vivo fluoroscopy. Spine. 2007; 32:527–536.
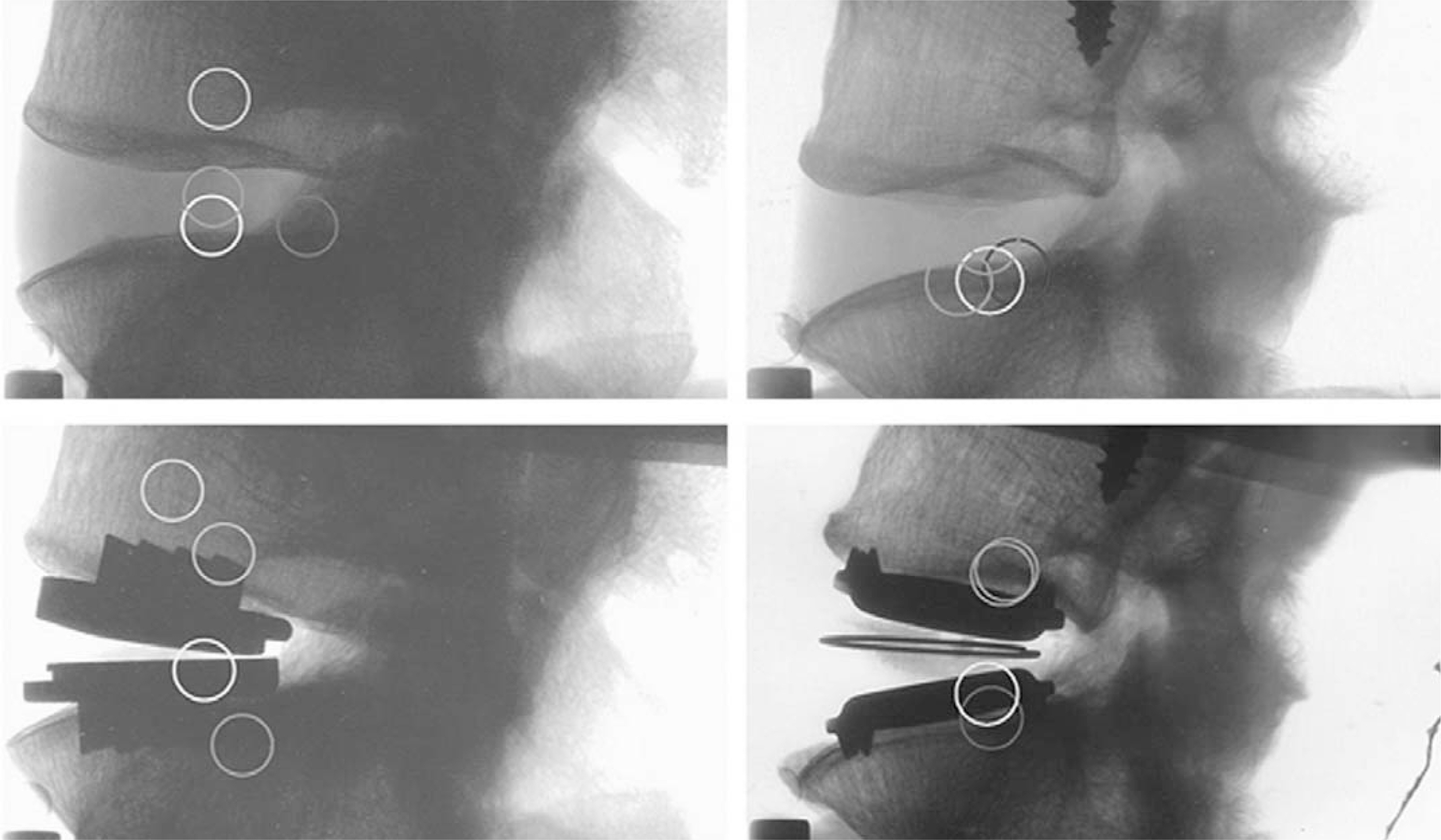
Article19). McAfee PC, Cunningham BW, Shimamoto N, et al. Biological ingrowth using a non-human primate model. Hochschuler SH, Buttner-Janz K, McAfee PC, editors. The SB Charite Artificial Disc. Berlin: Springer-Verlag;2003. p. 63–72.20). LeHuec JC, Kiaer T, Triesem T, Mathews H, Liu M, Eisermann L. Shock absorption in lumbar disc prosthesis: a preliminary mechanical study. J Spinal Disord Tech. 2003; 16:346–351.21). Rousseau MA, Bradford DS, Hadi TM, Pedersen KL, Lotz JC. The instant axis of rotation influences facet forces at L5/S1 during flexion/extension and lateral bending. Eur Spine J. 2006; 15:299–307.
Article22). Dooris AP, Goel VK, Grosland NM, Grosland NM, Gilbertson LG, Wilder DG. Load-Sharing Between anterior and posterior elements in a lumbar motion segment implanted with and artificial disc. Spine. 2001; 26:122–129.23). Lemaire JP, Skalli W, Lavaste F, et al. Intervertebral disc prosthesis. Results and prospects for the year 2000. Clin Orthop Relat Res. 1997; 337:64–76.
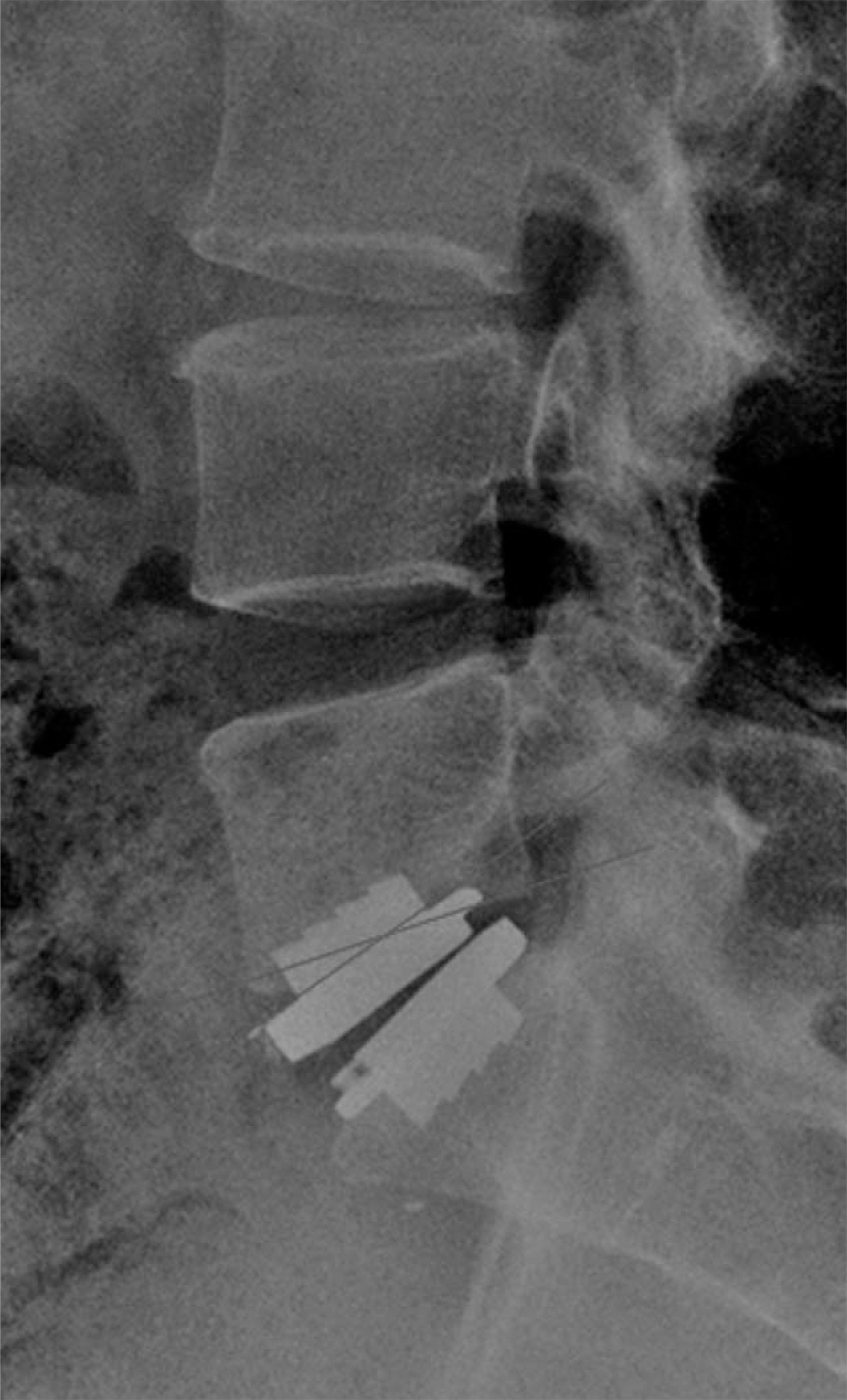
Article24). van Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite´ disc. J Spinal Disord Tech. 2003; 16:369–383.25). Shim CS, Lee S, Maeng DH, Lee SH. Vertical split fracture of the vertebral body following total disc replacement using ProDisc: report of two cases. J Spinal Disord Tech. 2005; 18:465–469.26). Kube R, Holt R, Majd M. Degeneration of lumbar disc and facets after disc arthroplasty: Results from 5 year follow-up in an IDE study. Presented at APSAS2008, Seoul, Korea. 2008.27). Lee CS, Chung SS, Lee JY. Changes of sagittal alignment of the lumbar spine after posterior spinal instrumentation in degenerative lumbar disease. J of Korean Musculoskeletal Tissue Transplant Soc. 2003; 3:36–43.28). Hedman MA, Kostuik JP, Fernie GR, et al. Design of an intervertebral disc prosthesis. Spine. 1991; 16:256–260.
Article29). Hallab N, Link HD, McAfee PC. Biomaterial optimization in total disc arthroplasty. Spine. 2003; 28:139–152.
Article30). Buttner-Janz K, Schellnack K, Zippel H. Biomechanics of the SB Charite Lumbar inter vertebral disc endoprosthesis. Int Orthop. 1989; 13:173–176.31). Kurtz SM, van Ooij A, Ross R, et al. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007; 7:12–21.
Article32). Kurtz SM, Peloza J Siskey R, Villarraga ML. Analysis of a retrieved polyethylene total disc replacement component. Spine J. 2005; 5:344–350.
Article33). Carragee EJ, Tanner CM, Yang B, Brito JL, Truong T. False-positive findings on lumbar discography. Reliability of subjective concordance assessment during provocative disc injection. Spine. 1999; 24:2542–2547.34). Siepe CJ, Korge A, Grochulla F, Mehren C, Mayer HM. Analysis of post-operative pain patterns following total lumbar disc replacement: results from fluoroscopically guided spine infiltrations. Eur Spine J. 2008; 17:44–56.
Article35). Bertagnoli R, Yue JJ, Fenk-Mayer A, Eerulkar J, Emerson JW. Treatment of symptomatic adjacent-segment degeneration after lumbar fusion with total disc arthroplasty by using the prodisc prosthesis: a prospective study with 2-year minimum follow up. J Neurosurg Spine. 2006; 4:91–97.
Article36). Bertagnoli R, Kumar S. Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J. 2002; 11:131–136.
Article37). Lee CS, Chung SS. Disease indication of Lumbar Total disc replacement. Presented at KOSAS meeting. 2005.38). Siepe CJ, Mayer HM, Heinz-Leisenheimer M, Korge A. Total lumbar disc replacement: different results for different levels. Spine. 2007; 32:782–790.39). Chin KR. Epidemiology of indications and contraindications to total disc replacement in an academic practice. Spine J. 2007; 7:392–398.
Article40). Huang RC, Lim MR, Girardi FP, Cammisa FP Jr. The prevalence of contraindications to total disc replacement in a cohort of lumbar surgical patients. Spine. 2004; 29:2538–2541.
Article41). Fras CI, Auerbach JD. Prevalence of Lumbar Total Disc Replacement Candidates in a Community-based Spinal Surgery Practice, J Spinal Disord Tech. 2008; 21:126–129.42). Zeegers WS, Bohnen LMLJ, Laaper M, Verhaegen MJA. Artificial disc replacement with the modular type SB Charite III: 2-year results in 50 prospectively studied patients. Eur Spine J. 1999; 8:210–217.43). Link HD, Keller A. Biomechanics of total disc replacement. Hochschuler SH, Buttner-Janz K, Mcafee PC, editors. The SB Charite Artificial Disc. Berlin: Springer-Verlag;2003. p. 33–52.
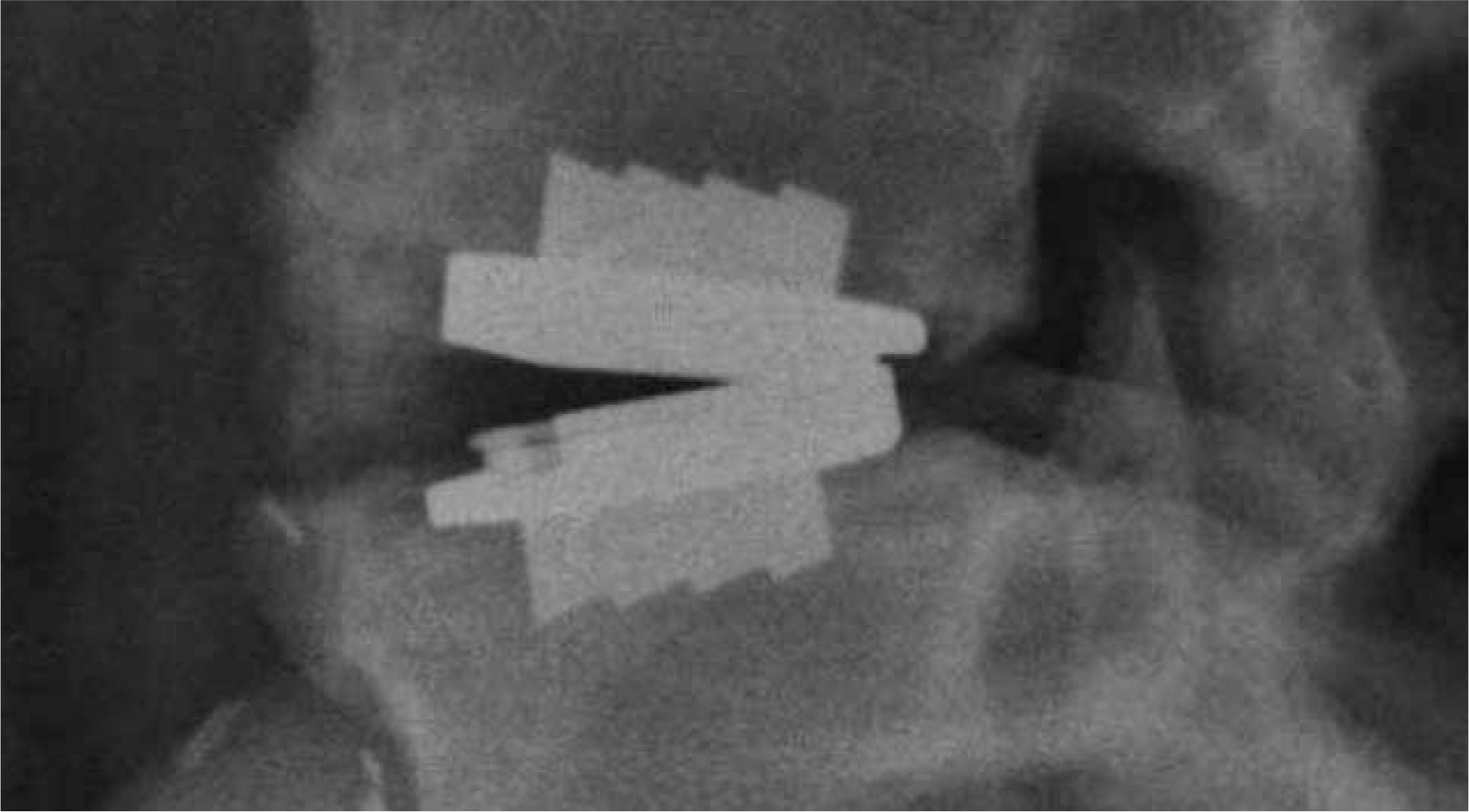
Article44). Lee CS, Chung SS, Ryu JW. The significance of angular mismatching between vertebral and prosthetic endplate. Presented at APSAS meeting. Jan. 2008. Seoul, Korea.45). Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007; 32:1155–1162.46). Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005; 30:1565–1575.47). Chung SS, Lee CS, Kang CS. Lumbar Total Disc Replacement Using ProDisc II: A Prospective Study With a 2-Year Minimum Follow-up. J Spinal Disord Tech. 2006; 19:411–415.48). David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITE artificial disc in 106 patients. Spine. 2007; 32:661–666.49). Putzier M, Funk JF, Schneider SV, et al. Charite´ total disc replacement-clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006; 15:183–195.50). Buttner-Janz K. Letter to the Editor concerning “Charite´ total disc replacement: clinical and radiographical results after an average follow-up of 17 years” (M. Putzier et al.). Eur Spine J. 2006; 15:510–513.51). Ahrens JE. An average six year clinical evaluation of the Link SB Charite intervertebral prosthesis. Final Report. Submitted to Brian Cameron President sales and marketing Link Spine Group. October 3. 1997.52). Thorpe PLP J, Licinia P. Osteolysis and complications associated with artificial disc replacement. Poster Annual Meeting of the Spine Society of Austrailia, April 17. 2004.53). McAfee PC, Geisler FH, Saiedy SS, et al. Revisability of the CHARITE artificial disc replacement: analysis of 688 patients enrolled in the U.S. IDE study of the CHARITE Artificial Disc. Spine. 2006; 31:1217–1226.54). van Ooij A, Kurtz SM, Stessels F, Noten H, van Rhijin L. Polyethylene wear debris and long-term clinical failure of the Charit? disc prosthesis: a study of 4 patients. Spine. 2007; 32:223–239.55). McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech. 2003; 16:384–389.
Article56). Tortolani PJ, Cunningham BW, Eng M, McAfee PC, Holsapple GA, Adams KA. Prevalence of heterotopic ossification following total disc replacement. A prospective, randomized study of two hundred and seventy-six patients. J Bone Joint Surg Am. 2007; 89:82–88.57). Lee CS, Chung SS, Ryu JW. Heterotopic ossification (HO) following lumbar total disc replacement. Presented at Korean Spine Society meeting, June. 2007.58). Arida D, Kabra A, Lowe C, Szafranski AM, Milestone D. Bio-medical marketing: The Charite´: Lessons in the Launch of a New Medical Device, June 2. 2006.59). Guyer RD, Tromanhauser SG, Regan JJ. An economic model of one-level lumbar arthroplasty versus fusion. Spine J. 2007; 7:558–562.
Article60). Decision Memo for Lumbar Artificial Disc Replacement (LADR) (CAG-00292R). August 14, 2007.61). Orthopedic Product News, Sep/Oct, 2007-2007 regulatory status.62). Devaine PA, Robinson EJ, Bourne RB, et al. Measurement of polyethylene wear in acetabular components inserted with and without cement. J Bone Joint Surg. Am. 1997; 79:682–689.63). David T. Revision of a Charite artificial disc 9.5 years in vivo to a new Charite artificial disc: case report and explant analysis. Eur Spine J. 2005; 26:Epub ahead of print.64). Punt IM, Visser VM, van Rhijin LW, et al. Complications and reoperations of the SB Charite´ lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008; 17:36–43.65). Pimenta L, Diaz RC, Guerrero LG. Charite´ lumbar artificial disc retrieval: use of a lateral minimally invasive technique. Technical note. J Neurosurg Spine. 2006; 5:556–561.66). Kotani Y, Abumi K, Shikinami Y, et al. Artificial intervertebral disc replacement using bioactive three-dimensional fabric desing, development, and preliminary animal study. Spine. 2002; 27:929–935.67). Lee CK, Langrana NA, Parsons JR, et al. Development of a prosthetic intervertebral disc. Spine. 1991; 16:253–255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lumbar Total Disc Replacement
- The Incidence of Potential Candidates for Total Disc Replacement among Lumbar and Cervical Fusion Patient Populations
- Perioperative Complications of Lumbar Total Disc Replacement
- Recycling of Cervical Artificial Disc for the Symptomatic Adjacent Segment Disorder Combined with Instability on Total Disc Replacement Area: A Case Report
- Clinical and Radiographic Analysis of Cervical Total Disc Replacement Versus Anterior Cervical fusion using Stand-alone Cage