J Korean Soc Spine Surg.
2004 Jun;11(2):77-82. 10.4184/jkss.2004.11.2.77.
Osseointegration with Ceramic Coated Implant
- Affiliations
-
- 1Department of Orthopaedic Surgery, College of Medicine, Yeungnam University, Taegu, Korea. mwahn@med.yu.ac.kr
- 2Department of Pathology College of Medicine, Yeungnam University, Taegu, Korea.
- 3Department of School of Advanced Materials Engineering, Yeungnam University, Taegu, Korea.
- KMID: 2097813
- DOI: http://doi.org/10.4184/jkss.2004.11.2.77
Abstract
- PURPOSE
This study was designed to clarify the osseointegration of the titanium screw coated with CMP, in regard to the time schedule, through the characteristic of early osseointegration.
MATERIALS AND METHODS
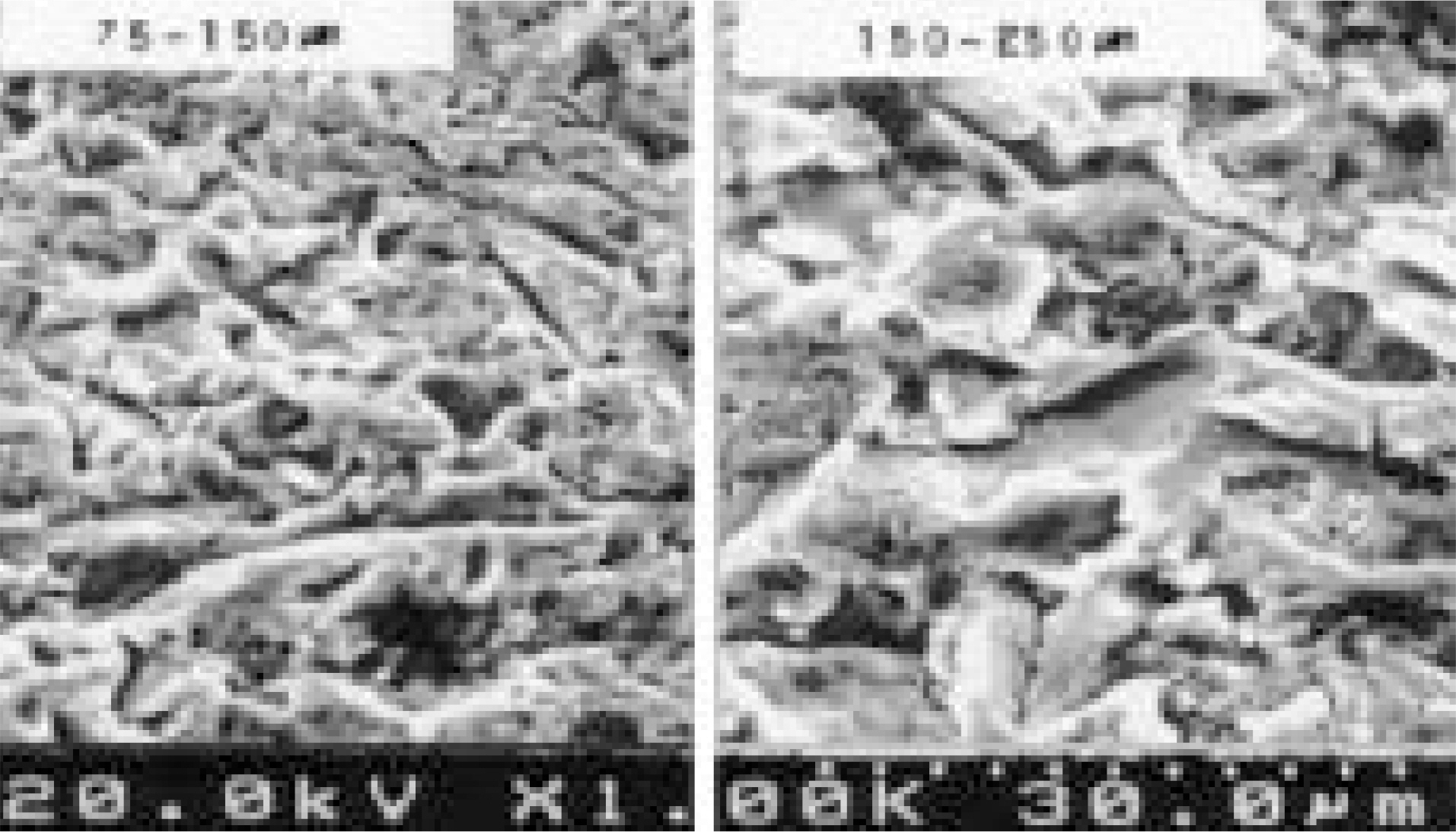
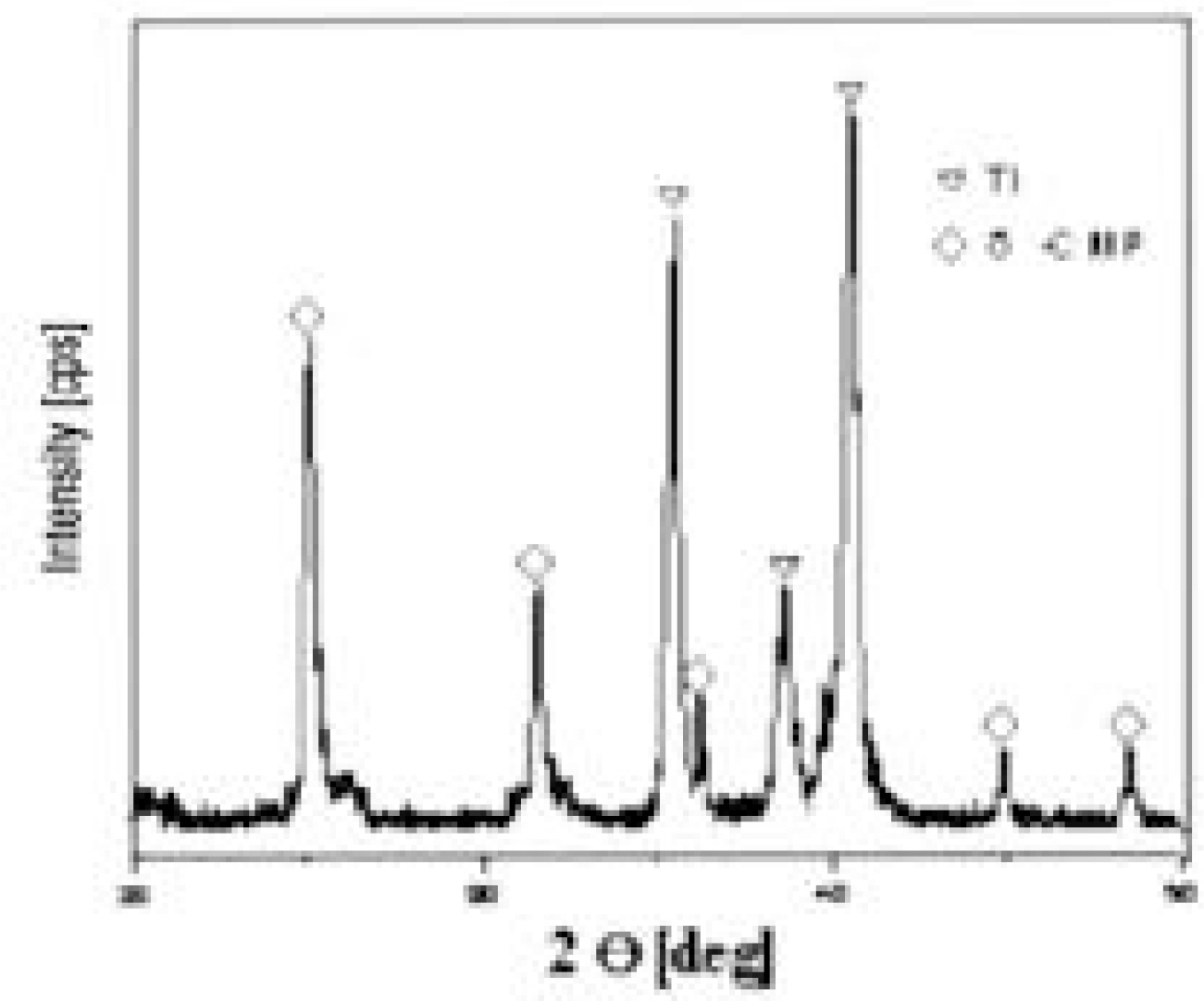
Mechanical, radiological and histomophometric measurements were performed in 28 rabbit tibial proximal metaphyseal cortical bone screws 6, 12, 26 and 52 weeks after surgery for the in vivo comparison of the osseointegration of titanium screws (3.75 mm diameter, 5 mm length) with different surface treatments: CMP coating group, with the sol-gel method (experimental group) and uncoated group (control group).
RESULTS
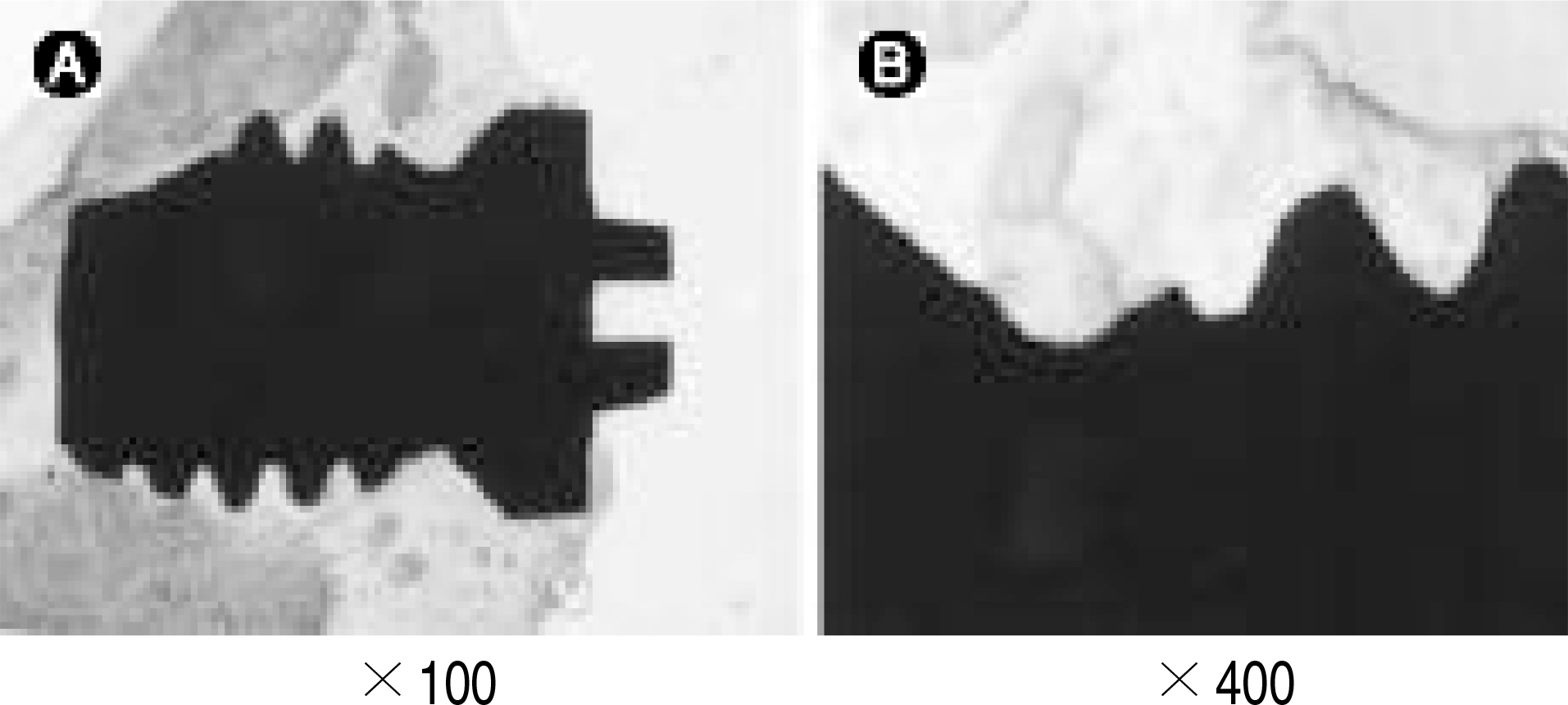
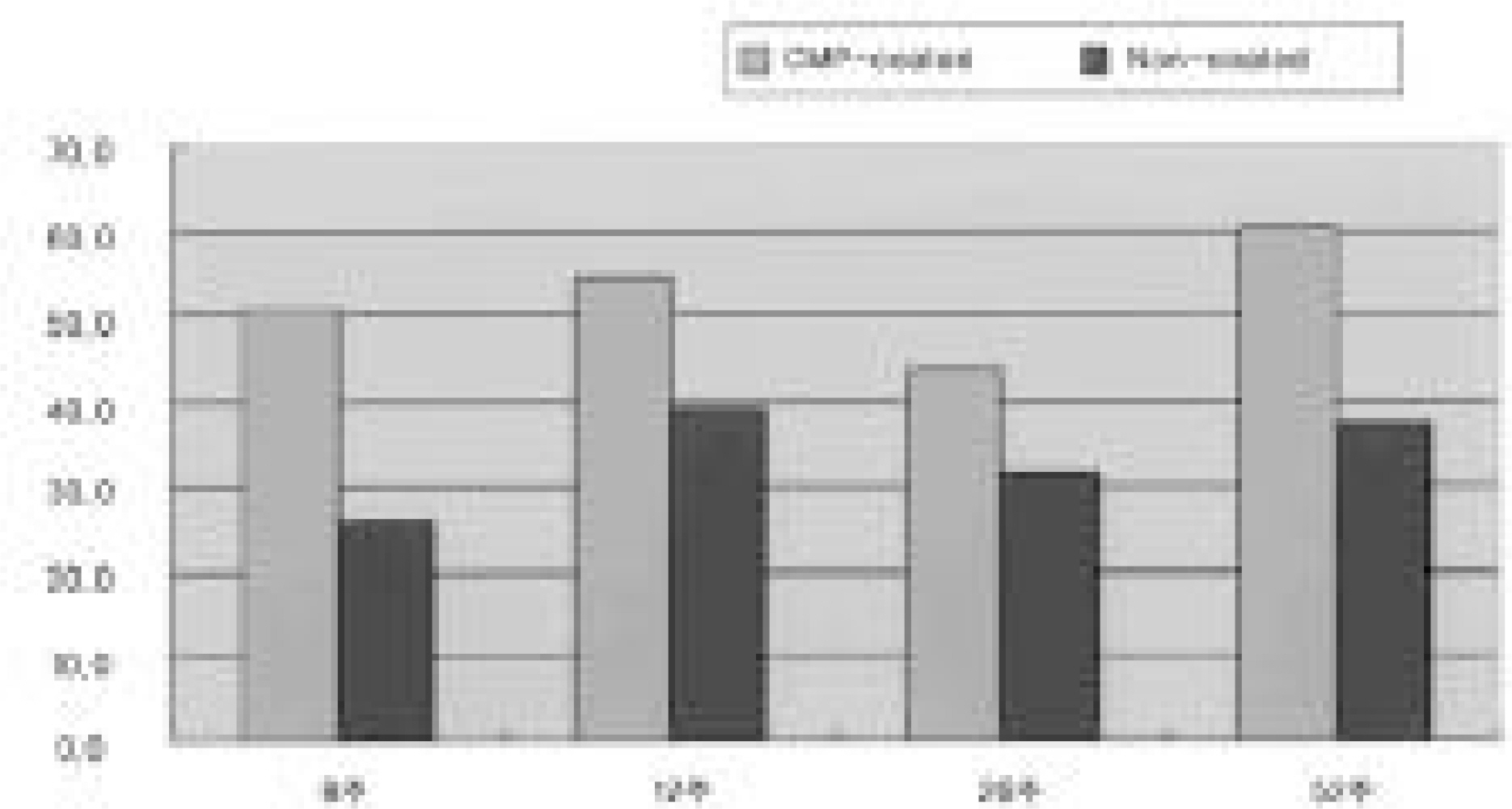
1. Radiology: There were no differences between the two groups without a radiolucent line or in regard to the time schedule. 2. Histology: There were no differences between the two groups without a fibrous tissue intervening surface or in regard to the time schedule. 3. Torque test: The test results for the CMP coated group were 1.5 times higher than those for the uncoated group, which was statistically meaningful, but there was no difference in regard to the time schedule.
CONCLUSION
CMP coating is an option to increase the osseointegration of the titanium screw.
Keyword
MeSH Terms
Figure
Reference
-
1). Gefen A. Optimizing the biomechanical compatibility of orthopedic screws for bone fracture fixation. Med Eng Phys. 2002; 24(5):337–347.
Article2). Kong YM, Kim DH, Kim HE, Heo SJ, Koak JY. Hydr oxyapatite-based composite for dental implants: An in vivo removal torque experiment. J Biomed Mater Res. 2002; 63(6):714–721.3). Sanden B, Olerud C, Petren-Mallmin M, Larsson S. Hydroxyapatite coating improves fixation of pedicle screws. A clinical study. J Bone Joint Surg Br. 2002; 84(3):387–391.4). Sanden B, Olerud C, Johansson C, Larsson S. I mpr oved bone-screw interface with hydroxyapatite coating: an in vivo study of loaded pedicle screws in sheep. Spine. 2001; 15:26. (24):2673–2678.5). Takeshita F, Ayukawa Y, Iyama S, Suetsugu T, Kido MA. A histologic evaluation of retrieved hydroxyapatite-coated blade-form implants using scanning electron, light, and confocal laser scanning microscopies. J Periodontol. 1996; 67(10):1034–1040.
Article6). Inadome T, Hayashi K, Nakashima Y, Tsumura H, Sugioka Y. Comparison of bone-implant interface shear strength of hydroxyapatite-coated and alumina-coated metal implants. J Biomed Mater Res. 1995; 29(1):19–24.
Article7). Albrektsson T, Branema PI, Hanson HA, Lindstrom J. Osseointegrated titanium implants. Acta Orthop Scand. 1981; 52:155–170.8). Johansson CG, Sennerby L, Albrektsson T. Remov al torque and histomorphometric study of bone tissue reactions to commercially pure titanium and vitallium implants. Int J Oral Maxillofac Implants. 1991; 6:437–441.9). Kasemo B. Biocompatibility of titanium implants: surface science aspects. J Prosthet Dent. 1983; 49:823–827.
Article10). Giavaresi G, Fini M, Chiesa R, et al. Osseointegration of sandblasted or anodised hydrothermally-treated titanium implants: mechanical, histomorphometric and bone hard -ness measurements. Int J Artif Organs. 2002; 25(8):806–813.11). Thomas KA, Cook SD. An evaluation of valuables influ -encing implant fixation by direct bone apposition. J Biomed Mater Res. 1985; 19:875–904.12). Lausma J, Mattsson L, Rolander U, Kasemo B. Chemical composition and morphology of titanium surface oxide. Mater Res Soc Symp Proc. 1986; 55:351–359.13). Hulshoff JE, Hayakawa T, van Dijk K, Leijdekkers-Govers AF, van der Waerden JP, Jansen JA. Mechani -cal and histologic evaluation of Ca-P plasma spray and magnetron sputter coated implants in trabecular bone of the goat. J Biomed Mater Res. 1997; 36.14). Lee IS, Kim DH, Kim HE, Jung YC, Han CH. Biologi -cal performance of calcium phosphate films formed on commercially pure Ti by electron-beam evaporation. Biomaterials. 2002; 23(2):609–615.15). Wolke JG, de Groot K, Jansen JA. In vivo dissolution behaviour of various RF magnetron sputtered Ca-P coatings. fifth world biomaterials congress, Toronto, Canada. 1996.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Teh Effect of Hydroxyapatite Coating on the Mechanical Strengths and Histologic Profiles of Porous Titanium Implants in Dogs
- A comparative experimental study of bone ingrowth and osseointegration in hydroxyapatite-coated vs. porous-coated implants
- The effect of fibronectin-coated implant on canine osseointegration
- Comparative Study on Osseointegration of Calcium Metaphosphate (CMP) Coated Implant to RBM Implant in the Femur of Rabbits
- Bone apposition on implants coated with calcium phosphate by ion beam assisted deposition in oversized drilled sockets: a histologic and histometric analysis in dogs