Yonsei Med J.
2015 Mar;56(2):355-361. 10.3349/ymj.2015.56.2.355.
Total Surface Area Is Useful for Differentiating between Aggressive and Favorable Multifocal Papillary Thyroid Carcinomas
- Affiliations
-
- 1Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. jhpath.sohn@samsung.com
- 2Department of Pathology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2070010
- DOI: http://doi.org/10.3349/ymj.2015.56.2.355
Abstract
- PURPOSE
The purpose of the present study was to identify more useful parameters for predicting behaviors of multifocal papillary thyroid carcinoma (PTC).
MATERIALS AND METHODS
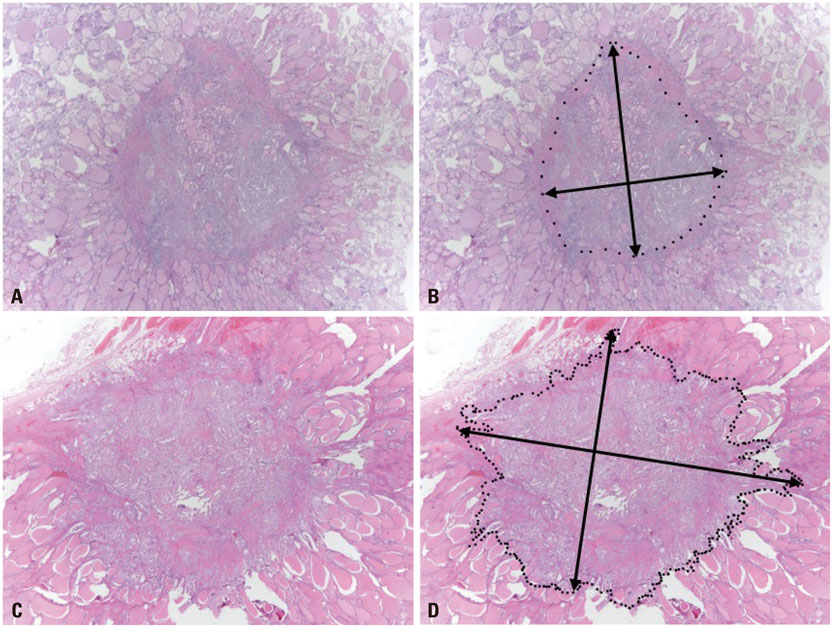
We investigated dominant tumor diameter (TD), total tumor diameter (TTD), and total surface area (TSA) in tumors from 588 patients and evaluated their usefulness as parameters for assessment of tumor behaviors in multifocal PTCs.
RESULTS
In 588 PTCs, tumor multifocality was found in 179 PTCs (30.4%). Multifocal tumors were significantly associated with extrathyroidal extension, lymph node metastasis, and higher tumor stage grouping (p<0.001, p<0.001, and p<0.001, respectively). The rates of nodal metastasis increased with greater TSA and TTD in PTCs. Multifocal papillary thyroid microcarcinomas (mPMCs) with TSA >3.14 cm2 had higher rates of nodal metastasis than mPMCs with TSA < or =3.14 cm2 (p=0.038); however, there was no significant difference between mPMCs with TTD >1.0 cm and with TTD < or =1.0 cm (p=0.325). In addition, nodal metastasis was more frequent in mPMCs with TSA >3.14 cm2 than in unifocal papillary thyroid microcarcinomas (uPMCs) (TD < or =1.0 cm) (p=0.002), but not overt unifocal PTCs (TD >1.0 cm) (p=0.244).
CONCLUSION
Our results suggest that mPMCs with TSA >3.14 cm2 show more aggressive behavior than uPMCs and mPMCs with TSA < or =3.14 cm2. TSA could be useful in distinguishing aggressive mPMCs from favorable cases.
MeSH Terms
Figure
Cited by 1 articles
-
Detection of Tumor Multifocality Is Important for Prediction of Tumor Recurrence in Papillary Thyroid Microcarcinoma: A Retrospective Study and Meta-Analysis
Jung-Soo Pyo, Jin Hee Sohn, Guhyun Kang
J Pathol Transl Med. 2016;50(4):278-286. doi: 10.4132/jptm.2016.03.29.
Reference
-
1. DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization Classification of Tumours. Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press;2004. p. 57–66.2. Shattuck TM, Westra WH, Ladenson PW, Arnold A. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. N Engl J Med. 2005; 352:2406–2412.
Article3. Siassakos D, Gourgiotis S, Moustafellos P, Dimopoulos N, Hadjiyannakis E. Thyroid microcarcinoma during thyroidectomy. Singapore Med J. 2008; 49:23–25.4. Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid. 2009; 19:707–716.
Article5. Ogilvie JB, Patel KN, Heller KS. Impact of the 2009 American Thyroid Association guidelines on the choice of operation for well-differentiated thyroid microcarcinomas. Surgery. 2010; 148:1222–1226.
Article6. Ciuffreda L, De Martino D, Bonfitto N, Scaramuzzi R. [Our experience on surgical treatment of papillary thyroid microcarcinoma]. G Chir. 2011; 32:41–44.7. Connor MP, Wells D, Schmalbach CE. Variables predictive of bilateral occult papillary microcarcinoma following total thyroidectomy. Otolaryngol Head Neck Surg. 2011; 144:210–215.
Article8. Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, et al. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol. 2013; 20:746–752.
Article9. Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009; 96:253–257.
Article10. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.
Article11. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag;2009. p. 87–100.12. Pyo JS, Kang G, Kim DH, Park C, Kim JH, Sohn JH. The prognostic relevance of psammoma bodies and ultrasonographic intratumoral calcifications in papillary thyroid carcinoma. World J Surg. 2013; 37:2330–2335.
Article13. Andea AA, Bouwman D, Wallis T, Visscher DW. Correlation of tumor volume and surface area with lymph node status in patients with multifocal/multicentric breast carcinoma. Cancer. 2004; 100:20–27.
Article14. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003; 98:31–40.
Article15. Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005; 103:2269–2273.
Article16. Pelizzo MR, Boschin IM, Toniato A, Piotto A, Bernante P, Pagetta C, et al. Papillary thyroid microcarcinoma (PTMC): prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol. 2006; 32:1144–1148.
Article17. Ghossein R, Ganly I, Biagini A, Robenshtok E, Rivera M, Tuttle RM. Prognostic factors in papillary microcarcinoma with emphasis on histologic subtyping: a clinicopathologic study of 148 cases. Thyroid. 2014; 24:245–253.
Article18. Katoh R, Sasaki J, Kurihara H, Suzuki K, Iida Y, Kawaoi A. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma. A clinicopathologic study of 105 consecutive patients. Cancer. 1992; 70:1585–1590.
Article19. Park SY, Park YJ, Lee YJ, Lee HS, Choi SH, Choe G, et al. Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer. 2006; 107:1831–1838.
Article20. Lin JD, Chao TC, Hsueh C, Kuo SF. High recurrent rate of multicentric papillary thyroid carcinoma. Ann Surg Oncol. 2009; 16:2609–2616.
Article21. Kim HJ, Kim NK, Choi JH, Kim SW, Jin SM, Suh S, et al. Radioactive iodine ablation does not prevent recurrences in patients with papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2013; 78:614–620.
Article22. Mazeh H, Samet Y, Hochstein D, Mizrahi I, Ariel I, Eid A, et al. Multifocality in well-differentiated thyroid carcinomas calls for total thyroidectomy. Am J Surg. 2011; 201:770–775.
Article23. Rodríguez-Cuevas S, Labastida-Almendaro S, Cortés-Arroyo H, López-Garza J, Barroso-Bravo S. Multifactorial analysis of survival and recurrences in differentiated thyroid cancer. Comparative evaluation of usefulness of AGES, MACIS, and risk group scores in Mexican population. J Exp Clin Cancer Res. 2002; 21:79–86.24. Voutilainen PE, Siironen P, Franssila KO, Sivula A, Haapiainen RK, Haglund CH. AMES, MACIS and TNM prognostic classifications in papillary thyroid carcinoma. Anticancer Res. 2003; 23:4283–4288.25. Appetecchia M, Scarcello G, Pucci E, Procaccini A. Outcome after treatment of papillary thyroid microcarcinoma. J Exp Clin Cancer Res. 2002; 21:159–164.26. Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008; 144:980–987.
Article27. Besic N, Pilko G, Petric R, Hocevar M, Zgajnar J. Papillary thyroid microcarcinoma: prognostic factors and treatment. J Surg Oncol. 2008; 97:221–225.
Article28. Pelizzo MR, Boschin IM, Toniato A, Pagetta C, Piotto A, Bernante P, et al. Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl Med Commun. 2004; 25:547–552.
Article29. Lee J, Rhee Y, Lee S, Ahn CW, Cha BS, Kim KR, et al. Frequent, aggressive behaviors of thyroid microcarcinomas in Korean patients. Endocr J. 2006; 53:627–632.
Article30. Page C, Biet A, Boute P, Cuvelier P, Strunski V. 'Aggressive papillary' thyroid microcarcinoma. Eur Arch Otorhinolaryngol. 2009; 266:1959–1963.
Article31. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008; 247:762–770.
Article32. Haymart MR, Cayo M, Chen H. Papillary thyroid microcarcinomas: big decisions for a small tumor. Ann Surg Oncol. 2009; 16:3132–3139.
Article33. Andea AA, Wallis T, Newman LA, Bouwman D, Dey J, Visscher DW. Pathologic analysis of tumor size and lymph node status in multifocal/multicentric breast carcinoma. Cancer. 2002; 94:1383–1390.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Concurrent Papillary and Medullary Thyroid Carcinomas Detected as Recurrent Medullary Carcinoma after Initial Surgery for Papillary Carcinoma
- The histological features of papillary thyroid carcinomas 1.5 cm and less in size
- Clinical Characteristics of Papillary Carcinomas with Other Benign Pathologies
- Oxyphilic Papillary Carcinoma of the Thyroid in Fine Needle Aspiration
- Prognostic Factors for Locally Invasive Papillary Thyroid Carcinomas