J Korean Med Sci.
2014 Dec;29(12):1610-1617. 10.3346/jkms.2014.29.12.1610.
Amyotrophic Lateral Sclerosis and Agricultural Environments: A Systematic Review
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 2Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea. leewj@korea.ac.kr
- KMID: 2069948
- DOI: http://doi.org/10.3346/jkms.2014.29.12.1610
Abstract
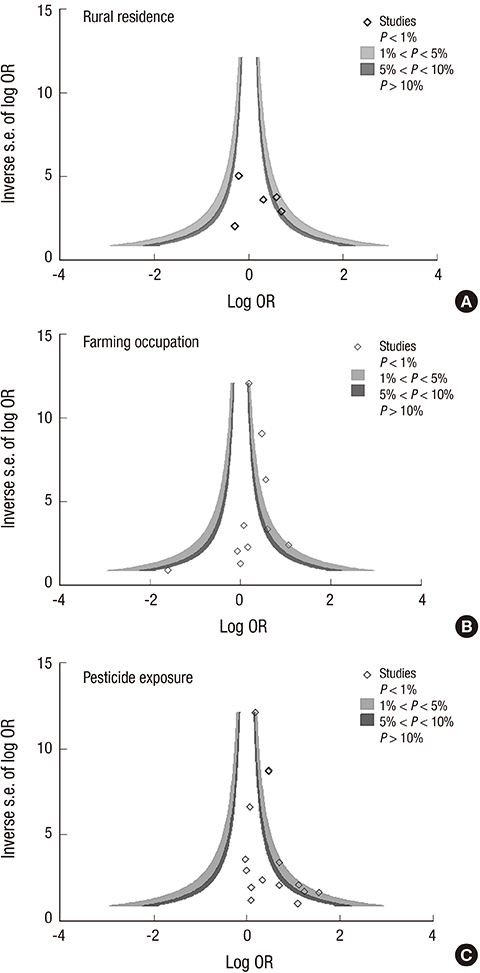
- The aim of this study was to examine the relationship between the risk of amyotrophic lateral sclerosis (ALS) and exposure to rural environments. Studies were identified through OVID MEDLINE and EMBASE search up to September 2013 using as keywords rural residence, farmers, and pesticide exposure. Twenty-two studies were included for this meta-analysis. Summary odds ratios (ORs) were calculated using random effect model by type of exposure index, and subgroup analyses were conducted according to study design, gender, region, case ascertainment, and exposure assessment. The risk of ALS was significantly increased with pesticide exposure (OR, 1.44; 95% CI, 1.22-1.70) and with farmers (OR, 1.42; 95% CI, 1.17-1.73), but was not significant with rural residence (OR, 1.25; 95% CI, 0.84-1.87). The risk estimates for subgroup analysis between pesticide exposure and ALS indicated a significant positive association with men (OR, 1.96), and in studies using El Escorial criteria for ALS definition (OR, 1.63) and expert judgment for pesticide exposure (OR, 2.04) as well. No significant publication bias was observed. Our findings support the association of pesticide exposure and an increased risk for ALS, stressing that the use of more specific exposure information resulted in more significant associations.
Keyword
MeSH Terms
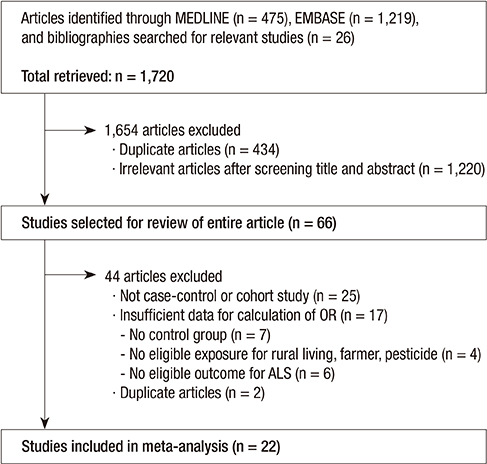
Figure
Reference
-
1. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet. 2011; 377:942–955.2. Ahmed A, Wicklund MP. Amyotrophic lateral sclerosis: what role does environment play? Neurol Clin. 2011; 29:689–711.3. Weisskopf MG, Morozova N, O'Reilly EJ, McCullough ML, Calle EE, Thun MJ, Ascherio A. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009; 80:558–561.4. Parrón T, Requena M, Hernández AF, Alarcón R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol Appl Pharmacol. 2011; 256:379–385.5. Keifer MC, Firestone J. Neurotoxicity of pesticides. J Agromedicine. 2007; 12:17–25.6. Morahan JM, Yu B, Trent RJ, Pamphlett R. A gene-environment study of the paraoxonase 1 gene and pesticides in amyotrophic lateral sclerosis. Neurotoxicology. 2007; 28:532–540.7. Choy S, Kim JW. A case of amyotrophic lateral sclerosis in a worker treating pesticide wastes. Korean J Occup Environ Med. 2011; 23:480–487.8. Bonvicini F, Marcello N, Mandrioli J, Pietrini V, Vinceti M. Exposure to pesticides and risk of amyotrophic lateral sclerosis: a population-based case-control study. Ann Ist Super Sanita. 2010; 46:284–287.9. McGuire V, Longstreth WT Jr, Nelson LM, Koepsell TD, Checkoway H, Morgan MS, van Belle G. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am J Epidemiol. 1997; 145:1076–1088.10. Morahan JM, Pamphlett R. Amyotrophic lateral sclerosis and exposure to environmental toxins: an Australian case-control study. Neuroepidemiology. 2006; 27:130–135.11. Savettieri G, Salemi G, Arcara A, Cassata M, Castiglione MG, Fierro B. A case-control study of amyotrophic lateral sclerosis. Neuroepidemiology. 1991; 10:242–245.12. Chancellor AM, Slattery JM, Fraser H, Warlow CP. Risk factors for motor neuron disease: a case-control study based on patients from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry. 1993; 56:1200–1206.13. Granieri E, Carreras M, Tola R, Paolino E, Tralli G, Eleopra R, Serra G. Motor neuron disease in the province of Ferrara, Italy, in 1964-1982. Neurology. 1988; 38:1604–1608.14. Gunnarsson LG, Bodin L, Söderfeldt B, Axelson O. A case-control study of motor neurone disease: its relation to heritability, and occupational exposures, particularly to solvents. Br J Ind Med. 1992; 49:791–798.15. Kamel F, Umbach DM, Bedlack RS, Richards M, Watson M, Alavanja MC, Blair A, Hoppin JA, Schmidt S, Sandler DP. Pesticide exposure and amyotrophic lateral sclerosis. Neurotoxicology. 2012; 33:457–462.16. Malek AM, Barchowsky A, Bowser R, Youk A, Talbott EO. Pesticide exposure as a risk factor for amyotrophic lateral sclerosis: a meta-analysis of epidemiological studies: pesticide exposure as a risk factor for ALS. Environ Res. 2012; 117:112–119.17. Das K, Nag C, Ghosh M. Familial, environmental, and occupational risk factors in development of amyotrophic lateral sclerosis. N Am J Med Sci. 2012; 4:350–355.18. Furby A, Beauvais K, Kolev I, Rivain JG, Sébille V. Rural environment and risk factors of amyotrophic lateral sclerosis: a case-control study. J Neurol. 2010; 257:792–798.19. Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005; 48:63–77.20. Kalfakis N, Vassilopoulos D, Voumvourakis C, Ndjeveleka M, Papageorgiou C. Amyotrophic lateral sclerosis in southern Greece: an epidemiologic study. Neuroepidemiology. 1991; 10:170–173.21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012.22. Pamphlett R. Exposure to environmental toxins and the risk of sporadic motor neuron disease: an expanded Australian case-control study. Eur J Neurol. 2012; 19:1343–1348.23. Qureshi MM, Hayden D, Urbinelli L, Ferrante K, Newhall K, Myers D, Hilgenberg S, Smart R, Brown RH, Cudkowicz ME. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler. 2006; 7:173–182.24. Burns CJ, Beard KK, Cartmill JB. Mortality in chemical workers potentially exposed to 2,4-dichlorophenoxyacetic acid (2,4-D) 1945-94: an update. Occup Environ Med. 2001; 58:24–30.25. Cruz DC, Nelson LM, McGuire V, Longstreth WT Jr. Physical trauma and family history of neurodegenerative diseases in amyotrophic lateral sclerosis: a population-based case-control study. Neuroepidemiology. 1999; 18:101–110.26. Fang F, Quinlan P, Ye W, Barber MK, Umbach DM, Sandler DP, Kamel F. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009; 117:1387–1392.27. Sutedja NA, Veldink JH, Fischer K, Kromhout H, Wokke JH, Huisman MH, Heederik DJ, Van den Berg LH. Lifetime occupation, education, smoking, and risk of ALS. Neurology. 2007; 69:1508–1514.28. Deapen DM, Henderson BE. A case-control study of amyotrophic lateral sclerosis. Am J Epidemiol. 1986; 123:790–799.29. Chió A, Meineri P, Tribolo A, Schiffer D. Risk factors in motor neuron disease: a case-control study. Neuroepidemiology. 1991; 10:174–184.30. Strickland D, Smith SA, Dolliff G, Goldman L, Roelofs RI. Amyotrophic lateral sclerosis and occupational history. A pilot case-control study. Arch Neurol. 1996; 53:730–733.31. Gunnarsson LG, Lindberg , Söderfeldt B, Axelson O. Amyotrophic lateral sclerosis in Sweden in relation to occupation. Acta Neurol Scand. 1991; 83:394–398.32. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1:293–299.33. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188.34. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.35. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.36. Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998; 316:61–66.37. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56:455–463.38. MacFarlane E, Glass D, Fritschi L. Is farm-related job title an adequate surrogate for pesticide exposure in occupational cancer epidemiology? Occup Environ Med. 2009; 66:497–501.39. Van Maele-Fabry G, Lantin AC, Hoet P, Lison D. Childhood leukaemia and parental occupational exposure to pesticides: a systematic review and meta-analysis. Cancer Causes Control. 2010; 21:787–809.40. Kauppinen TP, Mutanen PO, Seitsamo JT. Magnitude of misclassification bias when using a job-exposure matrix. Scand J Work Environ Health. 1992; 18:105–112.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amyotrophic Lateral Sclerosis Associated With CADASIL
- Syndrome of Progressive Bulbar Palsy in Amyotrophic Lateral Sclerosis: A Case Report
- Apraxia of Eyelid Closure and Motor Impersistence of Eyelid in a Patient with Amyotrophic Lateral Sclerosis
- Diagnosis and management of amyotrophic lateral sclerosis
- Psychosocial Responses and Quality of Life among Amyotrophic Lateral Sclerosis Patients and Their Caregivers