Obstet Gynecol Sci.
2015 Sep;58(5):340-345. 10.5468/ogs.2015.58.5.340.
Performance of Momguard, a new non-invasive prenatal testing protocol developed in Korea
- Affiliations
-
- 1Department of Obstetrics and Gynecology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. hswon@amc.seoul.kr
- 2LabGenomics Clinical Research Institute, LabGenomics, Seongnam, Korea.
- KMID: 2058456
- DOI: http://doi.org/10.5468/ogs.2015.58.5.340
Abstract
OBJECTIVE
To evaluate the performance of Momguard, non-invasive prenatal test (NIPT) for detecting trisomy (T) 21, T18, T13, and sex-chromosome abnormalities recently developed in Korea.
METHODS
This preliminary study formed part of a large prospective cohort study conducted at Asan Medical Center, Seoul, Korea. Only pregnant women who underwent both NIPT and confirmatory karyotyping were included in this study. NIPT results were compared with those of karyotype analyses.
RESULTS
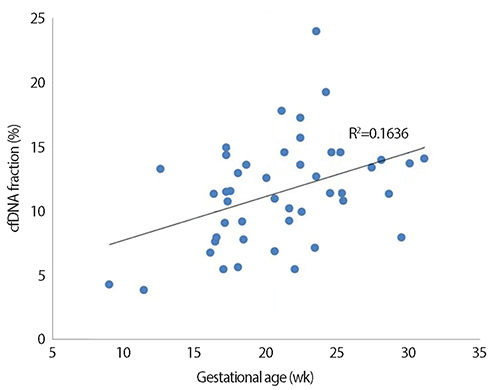
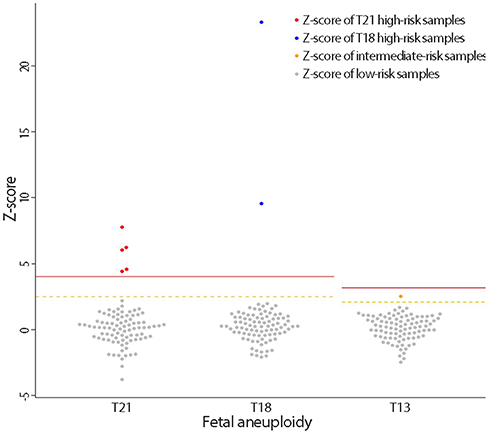
Among 93 eligible cases, NIPT results could not be obtained in one case due to a low fetal cell-free DNA fraction. Based on NIPT, eight cases of fetal aneuploidies, including T21 (n=5), T18 (n=2), and T13 (n=1), were identified. For T21 and T18, the sensitivity and specificity of NIPT were both 100%, with a false-positive and false-negative rate of 0% and a positive-predictive value of 100%. One patient classified as having intermediate risk for T13 by NIPT was confirmed to have T13 by karyotyping, and there were no false-negative cases. No cases of sex-chromosome anomalies were detected by NIPT or karyotyping during the study period.
CONCLUSION
Momguard is a reliable screening tool for detecting T21 and T18. For T13 and sex-chromosome anomalies, further prospective studies are necessary to confirm its utility.
MeSH Terms
Figure
Reference
-
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007; 110:1459–1467.2. Tabor A, Alfirevic Z. Update on procedure-related risks for prenatal diagnosis techniques. Fetal Diagn Ther. 2010; 27:1–7.3. Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005; 353:2001–2011.4. Nicolaides KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol. 2004; 191:45–67.5. Rozenberg P, Bussieres L, Chevret S, Bernard JP, Malagrida L, Cuckle H, et al. Screening for Down syndrome using first-trimester combined screening followed by second-trimester ultrasound examination in an unselected population. Am J Obstet Gynecol. 2006; 195:1379–1387.6. Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997; 350:485–487.7. Swanson A, Sehnert AJ, Bhatt S. Non-invasive prenatal testing: technologies, clinical assays and implementation strategies for women's healthcare practitioners. Curr Genet Med Rep. 2013; 1:113–121.8. Lo YM, Chan KC, Sun H, Chen EZ, Jiang P, Lun FM, et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med. 2010; 2:61ra91.9. Benn P, Cuckle H, Pergament E. Non-invasive prenatal testing for aneuploidy: current status and future prospects. Ultrasound Obstet Gynecol. 2013; 42:15–33.10. Gregg AR, Van den Veyver IB, Gross SJ, Madankumar R, Rink BD, Norton ME. Noninvasive prenatal screening by next-generation sequencing. Annu Rev Genomics Hum Genet. 2014; 15:327–347.11. Ehrich M, Deciu C, Zwiefelhofer T, Tynan JA, Cagasan L, Tim R, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011; 204:205.e1–205.e11.12. Norton ME, Brar H, Weiss J, Karimi A, Laurent LC, Caughey AB, et al. Non-Invasive Chromosomal Evaluation (NICE) Study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012; 207:137.e1–137.e8.13. Agarwal A, Sayres LC, Cho MK, Cook-Deegan R, Chandrasekharan S. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013; 33:521–531.14. Verweij EJ, de Boer MA, Oepkes D. Non-invasive prenatal testing for trisomy 13: more harm than good? Ultrasound Obstet Gynecol. 2014; 44:112–114.15. Daley R, Hill M, Chitty LS. Non-invasive prenatal diagnosis: progress and potential. Arch Dis Child Fetal Neonatal Ed. 2014; 99:F426–F430.16. Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W, Leung TY, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A. 2008; 105:20458–20463.17. Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013; 33:662–666.18. Song Y, Huang S, Zhou X, Jiang Y, Qi Q, Bian X, et al. Non-invasive prenatal testing for fetal aneuploidies in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2015; 45:55–60.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluating the results of the Momguard noninvasive prenatal test
- Prenatal screening for Down syndrome
- Clinical application of non-invasive prenatal testing using cell free fetal DNA
- Clinical Practice Guidelines for Prenatal Aneuploidy Screening and Diagnostic Testing from Korean Society of Maternal-Fetal Medicine:(2) Invasive Diagnostic Testing for Fetal Chromosomal Abnormalities
- The Effects of Prenatal Education on Primiparas' Perception of Delivery Experience, Self-Confidence and Satisfaction in Maternal Role Performance