J Bacteriol Virol.
2006 Jun;36(2):125-132. 10.4167/jbv.2006.36.2.125.
Evidence for the Presence of a cis-acting Element in the Coding Region of the Japanese Encephalitis Virus Capsid Protein
- Affiliations
-
- 1Department of Microbiology, College of Medicine and Medical Research Institute, Chungbuk National University, Cheongju, Korea. ymlee@chungbuk.ac.kr
- 2Research Institute of Veterinary Medicine, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 2055019
- DOI: http://doi.org/10.4167/jbv.2006.36.2.125
Abstract
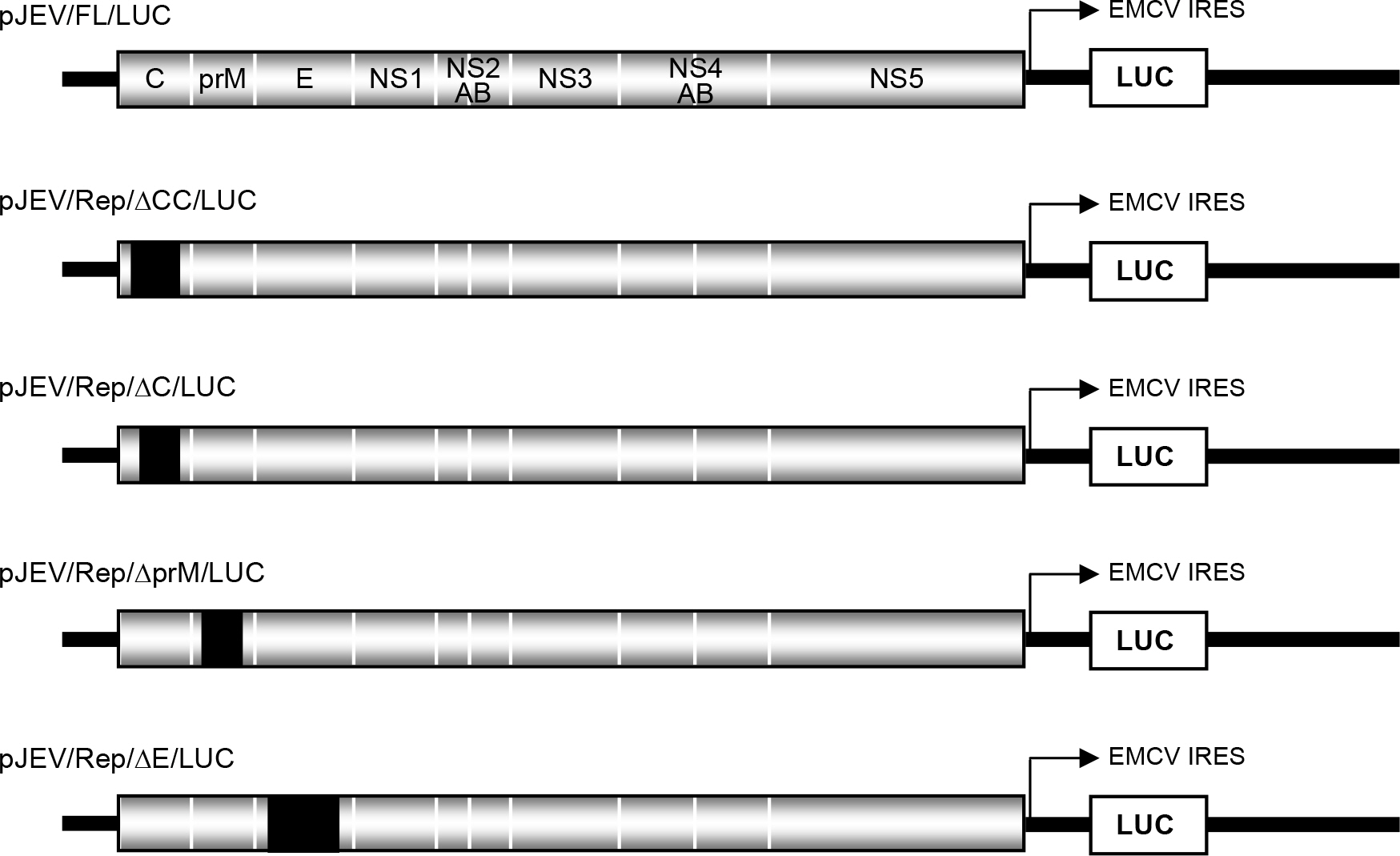
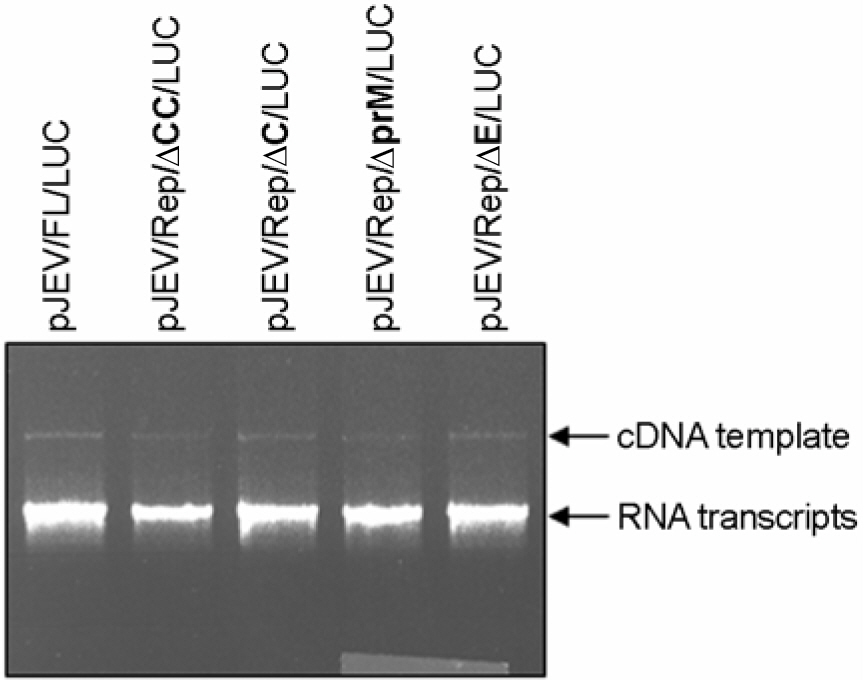
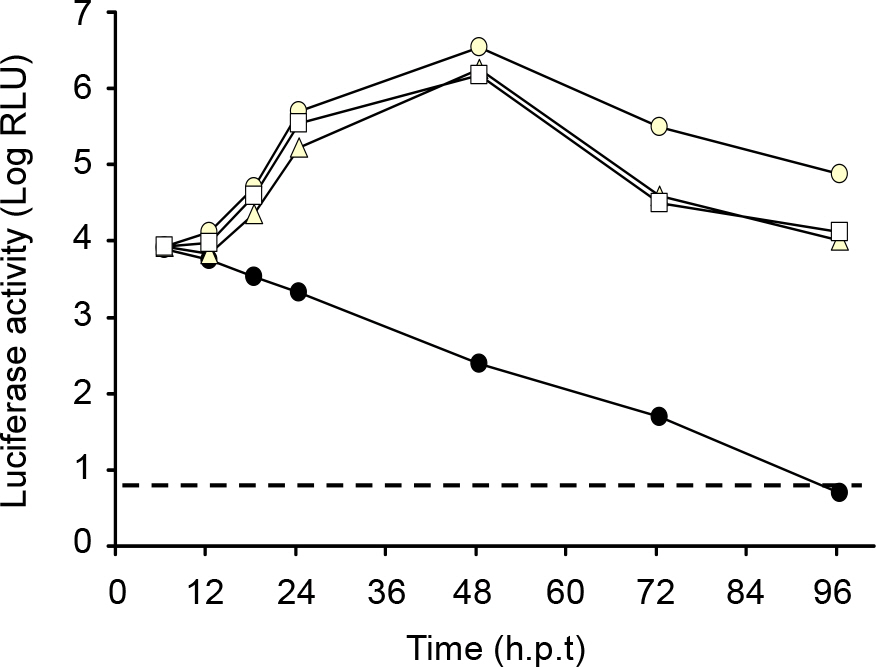
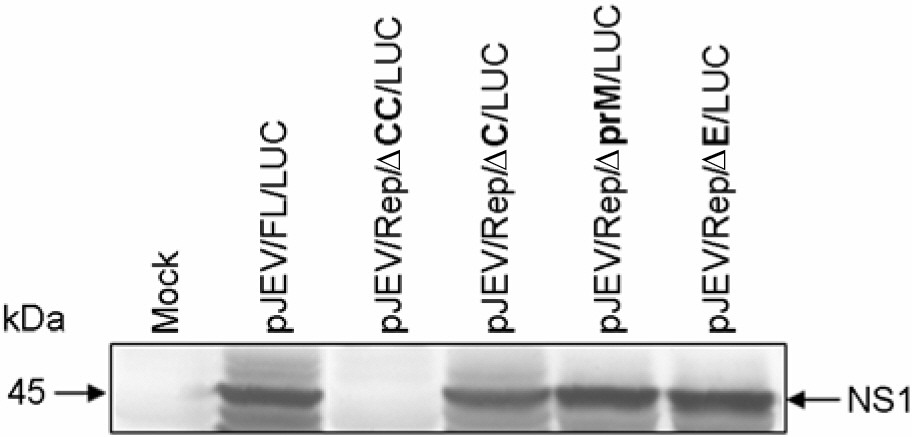
- Japanese encephalitis virus (JEV) is a member of mosquito-borne flaviviruses. To investigate whether there is a cis-acting genetic element in the coding region of the JEV C protein, which is required for viral replication, we generated four mutants by introducing a various size of deletions in each structural protein-coding region, designated as pJEV/Rep/deltaCC/LUC, pJEV/Rep/deltaC/LUC, pJEV/Rep/deltaprM/LUC, and pJEV/Rep/deltaE/LUC, of these, all replicons except for pJEV/Rep/deltaCC/LUC were competent in replication. Since pJEV/Rep/deltaCC/LUC is the same as pJEV/Rep/ deltaC/LUC except for an additional 5' deletion (nt 132~201) in the coding region of the C protein, this region appeared to be essential for RNA replication. This is consistent with the proposed cyclization sequence motif in the 5' region of the C gene, which has been recently shown to be required for replication in other mosquito-borne flaviviruses such as DV, YFV, KUN, and WNV. Thus, our results suggest that a cis-acting genetic element in the coding region of the JEV C protein may play an important role in RNA replication. This study will facilitate the current understanding of JEV RNA replication.
MeSH Terms
Figure
Reference
-
References
1). Ackermann M, Padmanabhan R. De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J. Biol. Chem. 276:39926–39937. 2001.
Article2). Alvarez DE, Lella Ezcurra AL, Fucito S, Gamarnik AV. Role of RNA structures present at the 3UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology. 339:200–212. 2005.
Article3). Blumenthal T, Carmichael GG. RNA replication: function and structure of Qbeta-replicase. Annu. Rev. Biochem. 48:525–548. 1979.
Article4). Burke DS, Monath TP. Flaviviruses. p. 1043–1125. In. Knipe D.M., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B, Straus S. E., editors(ed.).Fields virology. 4th ed.vol. 1. Lippincortt Williams & Wilkins;Philadelphia, Pa: 2001.5). Cahour A, Pletnev A, Vazielle-Falcoz M, Rosen L, Lai CJ. Growth-restricted dengue virus mutants containing deletions in the 5′ noncoding region of the RNA genome. Virology. 207:68–76. 1995.
Article6). Chambers TJ, Halm CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44:649–688. 1990.
Article7). Contreras R, Cherouter H, Degrave W, Fiers W. Simple, efficient in vitro synthesis of capped RNA useful for direct expression of cloned eukaryotic genes. Nucleic Acids Res. 10:6353–6362. 1989.8). Guirakhoo F, Monath TP. Immunoginicity, genetic stability, and protectiver efficacy of a recombinant, chimeric yellow fever-japanese encephalitis virus (chimerivax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology. 257:363–372. 1999.9). Hahn CS, Hahn YS, Rice CM, Lee E, Dalgarno L, Strauss EG, Strauss JH. Conserved elements in the 3untranslated region of flavivirus RNAs and potential cyclization sequences. J. Mol. Biol. 198:33–41. 1987.10). Herold J, Andino R. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol. Cell. 7:581–591. 2001.
Article11). Hewlett MJ, Pettersson RF, Baltimore D. Circular forms of Uukuniemi virion RNA: an electron microscopic study. J. Virol. 21:1085–1093. 1977.
Article12). Hofacker IL, Fekete M, Flamm C, Huynen MA, Rauscher S, Stolorz PE, Stadler PF. Automatic detection of conserved RNA structure elements in complete RNA virus genomes. Nucleic Acids Res. 26:3825–3836. 1998.
Article13). Hofacker IL, Fontana W, Stadler PF, Bonhoeffer S, Tacker M, Schuster P. Fast folding and comparison of RNA secondary structure. Monatsh. Chem. 125:167–188. 1994.14). Hofacker IL, Stadler PF. Automatic detection of conserved base pairing patterns in RNA virus genomes. Comput. Chem. 23:401–414. 1999.
Article15). Holden KL, Harris E. Enhancement of dengue virus translation:role of the 3untranslated region and the terminal 3stem-loop domain. Virology. 329:119–133. 2004.16). Hsu MT, Parvin JD, Gupta S, Krystal M, Palese P. Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle. Proc. Natl. Acad. Sci. USA. 84:8140–8144. 1987.
Article17). Khromykh AA, Meka H, Guyatt KJ, Westaway EG. Essential role of cyclization sequences in flavivirus RNA replication. J. Virol. 75:6719–6728. 2001.
Article18). Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the west nile virus responsible for an outbreak of encephalitis in the northeastern United states. Science. 286:2333–2337. 1999.
Article19). Lo MK, Tilgner M, Bernard KA, Shi PY. Functional analysis of mosquito-borne flavivirus conserved sequence elements within 3untranslated region of West Nile virus by use of a reporting replicon that differentiates between viral translation and RNA replication. J. Virol. 77:10004–10014. 2003.20). Mackenzie JM, Khromykh AA, Jones MK, Westaway EG. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology. 245:203–215. 1998.
Article21). Mandl CW, Ecker M, Holzmann H, Kunz C, Heinz FX. Infectious cDNA clones of tick-borne encephalitis virus European subtype prototypic strain Neudoerfl and high virulence strain Hypr. J. Gen. Virol. 78:1049–1057. 1997.
Article22). Markoff L. 5- and 3-noncoding regions in flavivirus RNA. Adv. Virus Res. 59:177–228. 2003.23). Rice CM. Flaviviridae: The viruses and their replication, p.931–960. In B. N. Fields, D. M. Knipe and P. M. Howley (ed), Fields Virology, Third ed, vol. 1. Lippincott-Raven Publishers, Philadelphia. 1996.24). Solomon T. Origin and evolution of Japanese encephalitis virus in southest asia. J. Virol. 77.5:3091–3098. 2003.25). Thiel HJ, Plagemann PGW, Moennig V. Pestiviruses. p. 1059–1073. In. Field B.N., Knipe D.M., Howley P.M., editors(ed),. Fields Virology. Raven Press;New York, N.Y.: 1996.26). Wells SE, Hillner PE, Vale RD, Sachs AB. Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell. 2:135–140. 1998.
Article27). Yun SI, Kim SY, Lee YM. Development and apolication of a reverse genetics system for Japanese encephalitis virus. J. Virol. 77(11):6450–6465. 2003.28). Yun SI, Kim SY, Choi WY, Nam JH, Ju YR, Park KY, Cho HW, Lee YM. Molecular characterization of the full-length genome of the Japanese encephalitis viral strain K87P39. Virus Res. 96(1–2):129–140. 2003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracellular Localization of the Japanese Encephalitis Virus Capsid Protein
- Expression and Purification of the Capsid Protein of the Japanese Encephalitis Virus and Production of its Polyclonal Antibody
- Haemagglutination inhibition antibodies of Japanese encephalitis virus to bats, Korea
- Analysis of the Three Dimensional Structure of Envelope Protein of the Japnes Encephalitis virus Isolated in Korea
- Expression of the Structural Proteins of Japanese Encephalitis Virus