Electrolyte Blood Press.
2008 Jun;6(1):1-8. 10.5049/EBP.2008.6.1.1.
Effects of Rosiglitazone on Heat Shock Protein and the Endothelin System in Deoxycorticosterone Acetate-Salt Hypertensive Rats
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. skimw@chonnam.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2052299
- DOI: http://doi.org/10.5049/EBP.2008.6.1.1
Abstract
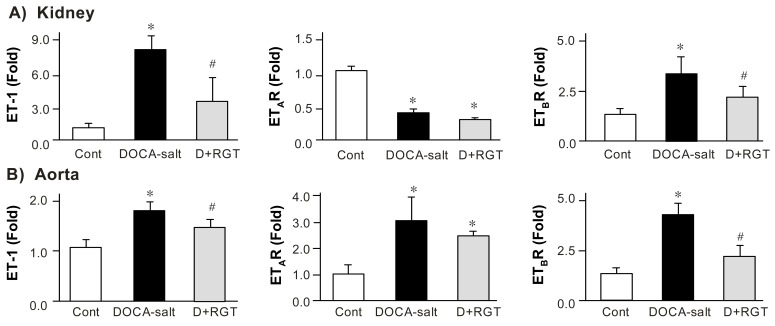
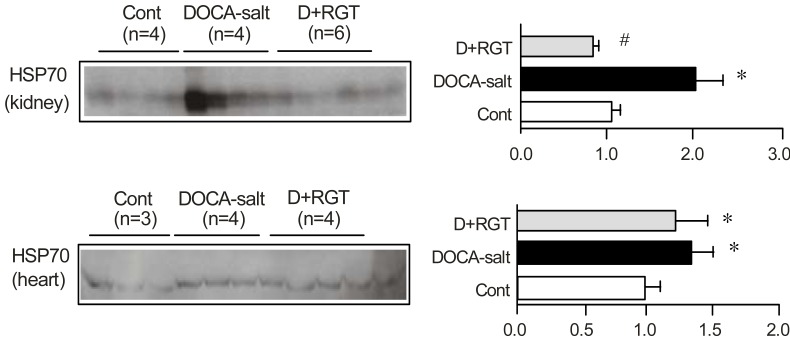
- The deoxycorticosterone acetate (DOCA)-salt rat is known as a model of volume dependent hypertension and characterized by increased cardiac endothelin-1 (ET-1) content. Recently, it has been reported that rosiglitazone (RGT), a peroxisome proliferator-activated subtype gamma receptor agonist, shows blood pressure lowering effect. We investigated whether DOCA-salt hypertension is associated with altered expression of heat shock proteins (HSP) and ET-1 in the heart, aorta, and kidney, and whether RGT changes HSP expression and ET-1 in association with its blood pressure lowering effect. Two weeks after the silastic DOCA (200 mg/kg) strips implantation, DOCA-salt rats were randomly divided to receive control diet with or without RGT (10 mg/kg/day) for another 2 weeks. The mRNA expression of ET-1 was determined by real time polymerase chain reaction. The expression of HSP was determined by semiquantitative immunoblotting. In DOCA-salt rats, systolic blood pressure was markedly increased, while creatinine clearance decreased. RGT treatment attenuated high blood pressure and decreased creatinine clearance in DOCA-salt rats. The mRNA expression of ET-1 was increased in DOCA-salt rats compared to controls, which was counteracted by RGT treatment. The protein expression of HSP70, HSP32, and HSP25 was increased in the kidney and heart in DOCA-salt rats, which was attenuated by RGT treatment in the kidney, but not in the heart. In conclusion, increased expression of ET-1 may play a role in the pathogenesis of hypertension in DOCA-salt rats, which was counteracted by the treatment of RGT. Up-regulation of HSP70, HSP32, and HSP25 in the kidney and heart may play a role in organ protection against a variety of stresses.
MeSH Terms
-
Animals
Aorta
Blood Pressure
Creatinine
Desoxycorticosterone
Diet
Dimethylpolysiloxanes
Endothelin-1
Endothelins
Heart
Heat-Shock Proteins
Hot Temperature
Hypertension
Immunoblotting
Kidney
Peroxisomes
Rats
Real-Time Polymerase Chain Reaction
RNA, Messenger
Thiazolidinediones
Up-Regulation
Creatinine
Desoxycorticosterone
Dimethylpolysiloxanes
Endothelin-1
Endothelins
Heat-Shock Proteins
RNA, Messenger
Thiazolidinediones
Figure
Reference
-
1. Laragh JH. Vasoconstriction-volume analysis for understanding and treating hypertension: the use of renin and aldosterone profiles. Am J Med. 1973; 55:261–274. PMID: 4355699.
Article2. Trinder D, Phillips PA, Risvanis J, Stephenson JM, Johnston CI. Regulation of vasopressin receptors in deoxycorticosterone acetate-salt hypertension. Hypertension. 1992; 20:569–574. PMID: 1398892.
Article3. Jeffries WB, Wang Y, Pettinger WA. Enhanced vasopressin (V2-receptor)-induced sodium retention in mineralocorticoid hypertension. Am J Physiol. 1988; 254:F739–F746. PMID: 2834967.
Article4. Kunes J, Nedvidek J, Zicha J. Vasopressin and water distribution in rats with DOCA-salt hypertension. J Hypertens Suppl. 1989; 7(Suppl 6):S204–S205. PMID: 2632718.
Article5. Miller RC, Pelton JT, Huggins JP. Endothelins--from receptors to medicine. Trends Pharmacol Sci. 1993; 14:54–60. PMID: 8480375.
Article6. Lariviere R, Thibault G, Schiffrin EL. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension. 1993; 21:294–300. PMID: 8478038.
Article7. Larouche I, Schiffrin EL. Cardiac microvasculature in DOCA-salt hypertensive rats : effect of endothelin ET(A) receptor antagonism. Hypertension. 1999; 34:795–801. PMID: 10523363.8. Hamet P, Malo D, Tremblay J. Increased transcription of a major stress gene in spontaneously hypertensive mice. Hypertension. 1990; 15:904–908. PMID: 2351441.
Article9. Ishizaka N, Aizawa T, Ohno M, Usui Si S, Mori I, Tang SS, et al. Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension. 2002; 39:122–128. PMID: 11799090.
Article10. Aizawa T, Ishizaka N, Taguchi J, Nagai R, Mori I, Tang SS, et al. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats : possible role in renoprotection. Hypertension. 2000; 35:800–806. PMID: 10720598.11. Xu Q, Li DG, Holbrook NJ, Udelsman R. Acute hypertension induces heat-shock protein 70 gene expression in rat aorta. Circulation. 1995; 92:1223–1229. PMID: 7648669.
Article12. Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999; 274:18947–18956. PMID: 10383393.13. Botros FT, Schwartzman ML, Stier CT Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005; 68:2745–2755. PMID: 16316349.
Article14. Day C. Thiazolidinediones: a new class of antidiabetic drugs. Diabet Med. 1999; 16:179–192. PMID: 10227562.
Article15. Dubey RK, Zhang HY, Reddy SR, Boegehold MA, Kotchen TA. Pioglitazone attenuates hypertension and inhibits growth of renal arteriolar smooth muscle in rats. Am J Physiol. 1993; 265:R726–R732. PMID: 8238439.
Article16. Gerber P, Lubben G, Heusler S, Dodo A. Effects of pioglitazone on metabolic control and blood pressure: a randomised study in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2003; 19:532–539. PMID: 14594526.
Article17. Raji A, Seely EW, Bekins SA, Williams GH, Simonson DC. Rosiglitazone improves insulin sensitivity and lowers blood pressure in hypertensive patients. Diabetes Care. 2003; 26:172–178. PMID: 12502676.
Article18. Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, et al. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARgamma on vascular endothelial function. Biochem Biophys Res Commun. 1999; 254:757–763. PMID: 9920814.19. Artwohl M, Holzenbein T, Furnsinn C, Freudenthaler A, Huttary N, Waldhausl WK, et al. Thiazolidinediones inhibit apoptosis and heat shock protein 60 expression in human vascular endothelial cells. Thromb Haemost. 2005; 93:810–815. PMID: 15886792.
Article20. Bae EH, Kim IJ, Park JW, Ma SK, Choi KC, Lee JU, et al. Altered regulation of renin-angiotensin, endothelin and natriuretic peptide systems in rat kidney with chronic unilateral ureteral obstruction. Urol Int. 2007; 79:170–176. PMID: 17851289.
Article21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. PMID: 11846609.22. Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005; 43:19–29. PMID: 15955745.
Article23. Li JS, Turgeon A, Schiffrin EL. Effect of chronic treatment with two different ET(A) selective endothelin receptor antagonists on blood pressure and small artery structure of deoxycorticosterone acetate (DOCA)-salt hypertensive rats. Am J Hypertens. 1998; 11:554–562. PMID: 9633791.
Article24. Schiffrin EL, Sventek P, Li JS, Turgeon A, Reudelhuber T. Antihypertensive effect of an endothelin receptor antagonist in DOCA-salt spontaneously hypertensive rats. Br J Pharmacol. 1995; 115:1377–1381. PMID: 8564194.
Article25. Hughes AK, Stricklett PK, Padilla E, Kohan DE. Effect of reactive oxygen species on endothelin-1 production by human mesangial cells. Kidney Int. 1996; 49:181–189. PMID: 8770966.
Article26. Kohan DE, Fiedorek FT Jr. Endothelin synthesis by rat inner medullary collecting duct cells. J Am Soc Nephrol. 1991; 2:150–155. PMID: 1954327.
Article27. Terada Y, Tomita K, Nonoguchi H, Marumo F. Different localization of two types of endothelin receptor mRNA in microdissected rat nephron segments using reverse transcription and polymerase chain reaction assay. J Clin Invest. 1992; 90:107–112. PMID: 1321837.
Article28. Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, et al. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993; 91:1367–1373. PMID: 7682570.
Article29. Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, et al. The heart in hypertension. N Engl J Med. 1992; 327:998–1008. PMID: 1518549.
Article30. Smoyer WE, Ransom R, Harris RC, Welsh MJ, Lutsch G, Benndorf R. Ischemic acute renal failure induces differential expression of small heat shock proteins. J Am Soc Nephrol. 2000; 11:211–221. PMID: 10665928.
Article31. Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997; 170:130–137. PMID: 9009141.
Article32. Komatsuda A, Wakui H, Oyama Y, Imai H, Miura AB, Itoh H, et al. Overexpression of the human 72 kDa heat shock protein in renal tubular cells confers resistance against oxidative injury and cisplatin toxicity. Nephrol Dial Transplant. 1999; 14:1385–1390. PMID: 10382997.
Article33. Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992; 90:267–270. PMID: 1634613.
Article34. Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000; 278:F726–F736. PMID: 10807584.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Upregulation of Heat Shock Proteins in the Kidney in Hypertension
- Role of tyrosine kinases in vascular contraction in deoxycorticosterone acetate-salt hypertensive rats
- Effects of Centrally Administered Losartan on Deoxycorticosterone-salt Hypertension Rats
- Tissue-specific regulation of angiotensinogen and angiotensin II receptor gene expression in deoxycorticosterone acetate-salt hypertensive rats
- Coordinate expression of renin and cyclooxygenase-2 in rats with two-kidney, one clip and deoxycorticosterone acetate-salt hypertension