J Periodontal Implant Sci.
2014 Oct;44(5):242-250. 10.5051/jpis.2014.44.5.242.
Early bone healing onto implant surface treated by fibronectin/oxysterol for cell adhesion/osteogenic differentiation: in vivo experimental study in dogs
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs
- 2Department of Periodontology, Kyung Hee University School of Dentistry, Seoul, Korea.
- 3Department of Dental Biomaterials Science, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea.
- 4Atomic-Scale Surface Science Research Center, Yonsei University, Seoul, Korea.
- KMID: 2027822
- DOI: http://doi.org/10.5051/jpis.2014.44.5.242
Abstract
- PURPOSE
This study aimed to evaluate the effects of fibronectin and oxysterol immobilized on machined-surface dental implants for the enhancement of cell attachment and osteogenic differentiation, on peri-implant bone healing in the early healing phase using an experimental model in dogs.
METHODS
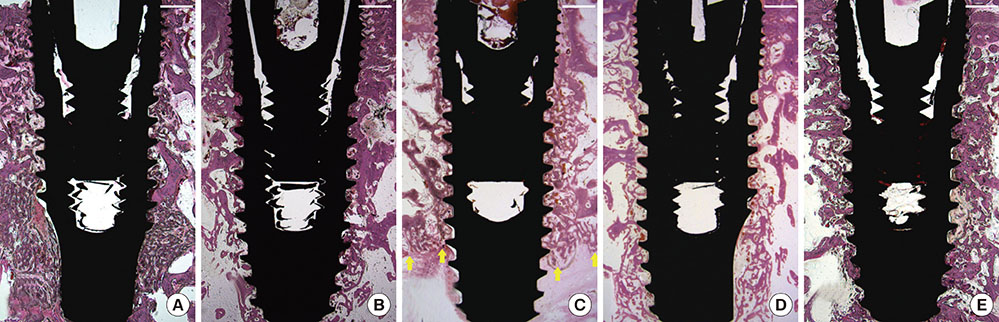
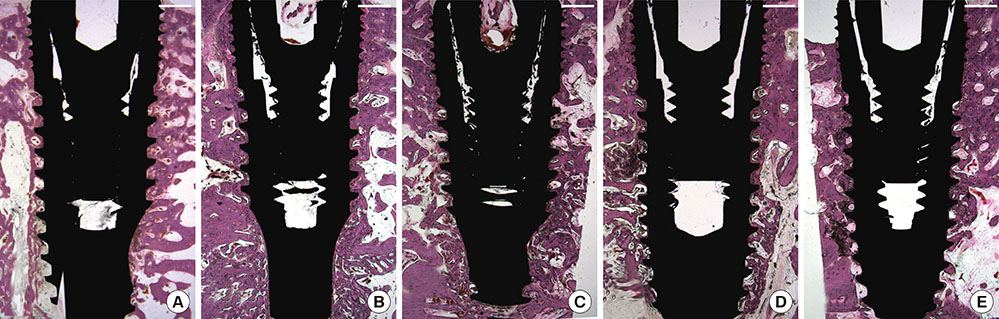
Five types of dental implants were installed at a healed alveolar ridge in five dogs: a machined-surface implant (MI), apatite-coated MI (AMI), fibronectin-loaded AMI (FAMI), oxysterol-loaded AMI (OAMI), and sand-blasted, large-grit, acid-etched surface implant (SLAI). A randomly selected unilateral ridge was observed for 2 weeks, and the contralateral ridge for a 4-week period. Histologic and histometric analyses were performed for the bone-to-implant contact proportion (BIC) and bone density around the dental implant surface.
RESULTS
Different bone healing patterns were observed according to the type of implant surface 2 weeks after installation; newly formed bone continuously lined the entire surfaces in specimens of the FAMI and SLAI groups, whereas bony trabecula from adjacent bone tissue appeared with minimal new bone lining onto the surface in the MI, AMI, and OAMI groups. Histometric results revealed a significant reduction in the BIC in MI, AMI, and OAMI compared to SLAI, but FAMI demonstrated a comparable BIC with SLAI. Although both the BIC and bone density increased from a 2- to 4-week healing period, bone density showed no significant difference among any of the experimental and control groups.
CONCLUSIONS
A fibronectin-coated implant surface designed for cell adhesion could increase contact osteogenesis in the early bone healing phase, but an oxysterol-coated implant surface designed for osteoinductivity could not modify early bone healing around implants in normal bone physiology.
MeSH Terms
Figure
Reference
-
1. Choi AH, Ben-Nissan B, Matinlinna JP, Conway RC. Current perspectives: calcium phosphate nanocoatings and nanocomposite coatings in dentistry. J Dent Res. 2013; 92:853–859.2. Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration: a literature review. Int J Oral Maxillofac Implants. 2000; 15:675–690.3. Scacchi M, Merz BR, Schar AR. The development of the ITI DENTAL IMPLANT SYSTEM. Part 2: 1998-2000: Steps into the next millennium. Clin Oral Implants Res. 2000; 11:Suppl 1. 22–32.4. Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998; 40:1–11.
Article5. Davies JE. Mechanisms of endosseous integration. Int J Prosthodont. 1998; 11:391–401.6. Weber HP, Fiorellini JP. The biology and morphology of the implant-tissue interface. Alpha Omegan. 1992; 85:61–64.7. Albrektsson T, Branemark PI, Hansson HA, Lindstrom J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–170.
Article8. Rahal MD, Branemark PI, Osmond DG. Response of bone marrow to titanium implants: osseointegration and the establishment of a bone marrow-titanium interface in mice. Int J Oral Maxillofac Implants. 1993; 8:573–579.9. Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003; 67:932–949.
Article10. Ericsson I, Johansson CB, Bystedt H, Norton MR. A histomorphometric evaluation of bone-to-implant contact on machine-prepared and roughened titanium dental implants: a pilot study in the dog. Clin Oral Implants Res. 1994; 5:202–206.
Article11. Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000; 84:522–534.
Article12. Buser D, Nydegger T, Oxland T, Cochran DL, Schenk RK, Hirt HP, et al. Interface shear strength of titanium implants with a sandblasted and acid-etched surface: a biomechanical study in the maxilla of miniature pigs. J Biomed Mater Res. 1999; 45:75–83.
Article13. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants: a histomorphometric study in miniature pigs. J Biomed Mater Res. 1991; 25:889–902.
Article14. Park JY, Gemmell CH, Davies JE. Platelet interactions with titanium: modulation of platelet activity by surface topography. Biomaterials. 2001; 22:2671–2682.
Article15. Cochran DL, Buser D, ten Bruggenkate CM, Weingart D, Taylor TM, Bernard JP, et al. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: early results from clinical trials on ITI SLA implants. Clin Oral Implants Res. 2002; 13:144–153.
Article16. Lai HC, Zhuang LF, Liu X, Wieland M, Zhang ZY, Zhang ZY. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J Biomed Mater Res A. 2010; 93:289–296.
Article17. Wall I, Donos N, Carlqvist K, Jones F, Brett P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone. 2009; 45:17–26.
Article18. Qu Z, Rausch-Fan X, Wieland M, Matejka M, Schedle A. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J Biomed Mater Res A. 2007; 82:658–668.
Article19. Li Y, Lee IS, Cui FZ, Choi SH. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium. Biomaterials. 2008; 29:2025–2032.
Article20. Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007; 23:844–854.
Article21. Kim S, Myung WC, Lee JS, Cha JK, Jung UW, Yang HC, et al. The effect of fibronectin-coated implant on canine osseointegration. J Periodontal Implant Sci. 2011; 41:242–247.
Article22. Hilbig H, Kirsten M, Rupietta R, Graf HL, Thalhammer S, Strasser S, et al. Implant surface coatings with bone sialoprotein, collagen, and fibronectin and their effects on cells derived from human maxillar bone. Eur J Med Res. 2007; 12:6–12.23. Chen C, Lee IS, Zhang SM, Yang HC. Biomimetic apatite formation on calcium phosphate-coated titanium in Dulbecco's phosphate-buffered saline solution containing CaCl(2) with and without fibronectin. Acta Biomater. 2010; 6:2274–2281.
Article24. Gorbahn M, Klein MO, Lehnert M, Ziebart T, Brullmann D, Koper I, et al. Promotion of osteogenic cell response using quasicovalent immobilized fibronectin on titanium surfaces: introduction of a novel biomimetic layer system. J Oral Maxillofac Surg. 2012; 70:1827–1834.
Article25. Leknes KN, Yang J, Qahash M, Polimeni G, Susin C, Wikesjo UM. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: radiographic observations. Clin Oral Implants Res. 2008; 19:1027–1033.
Article26. Wikesjo UM, Qahash M, Polimeni G, Susin C, Shanaman RH, Rohrer MD, et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol. 2008; 35:1001–1010.
Article27. Son KM, Park HC, Kim NR, Lee IS, Yang HC. Enhancement of the ALP activity of C3H10T1/2 cells by the combination of an oxysterol and apatite. Biomed Mater. 2010; 5:044107.
Article28. Stappenbeck F, Xiao W, Epperson M, Riley M, Priest A, Huang D, et al. Novel oxysterols activate the Hedgehog pathway and induce osteogenesis. Bioorg Med Chem Lett. 2012; 22:5893–5897.
Article29. Aghaloo TL, Amantea CM, Cowan CM, Richardson JA, Wu BM, Parhami F, et al. Oxysterols enhance osteoblast differentiation in vitro and bone healing in vivo. J Orthop Res. 2007; 25:1488–1497.
Article30. Johnson JS, Meliton V, Kim WK, Lee KB, Wang JC, Nguyen K, et al. Novel oxysterols have pro-osteogenic and anti-adipogenic effects in vitro and induce spinal fusion in vivo. J Cell Biochem. 2011; 112:1673–1684.
Article31. Esposito M, Murray-Curtis L, Grusovin MG, Coulthard P, Worthington HV. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database Syst Rev. 2007; (4):CD003815.
Article32. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992; 69:11–25.
Article33. Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010; 11:633–643.
Article34. Abrahamsson I, Berglundh T, Linder E, Lang NP, Lindhe J. Early bone formation adjacent to rough and turned endosseous implant surfaces: an experimental study in the dog. Clin Oral Implants Res. 2004; 15:381–392.
Article35. Piattelli A, Manzon L, Scarano A, Paolantonio M, Piattelli M. Histologic and histomorphometric analysis of the bone response to machined and sandblasted titanium implants: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1998; 13:805–810.36. Li D, Ferguson SJ, Beutler T, Cochran DL, Sittig C, Hirt HP, et al. Biomechanical comparison of the sandblasted and acid-etched and the machined and acid-etched titanium surface for dental implants. J Biomed Mater Res. 2002; 60:325–332.
Article37. Amantea CM, Kim WK, Meliton V, Tetradis S, Parhami F. Oxysterol-induced osteogenic differentiation of marrow stromal cells is regulated by Dkk-1 inhibitable and PI3-kinase mediated signaling. J Cell Biochem. 2008; 105:424–436.
Article38. Kha HT, Basseri B, Shouhed D, Richardson J, Tetradis S, Hahn TJ, et al. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J Bone Miner Res. 2004; 19:830–840.
Article39. Choi Y, Lee JS, Kim YJ, Kim MS, Choi SH, Cho KS, et al. Recombinant human bone morphogenetic protein-2 stimulates the osteogenic potential of the Schneiderian membrane: a histometric analysis in rabbits. Tissue Eng Part A. 2013; 19:1994–2004.
Article40. Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011; 17:1389–1399.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of fibronectin-coated implant on canine osseointegration
- Bone Healing around Screw-shaped Titanium Implants with Three Different Surface Topographies
- Effect of Recombinant Fragment of Fibronectin on the Cellular Functions of Human Bone Marrow-Derived Mesenchymal Stem Cells
- AN EXPERIMENTAL STUDY OF PLATELET-DERIVED GROWTH FACTOR ABOUT BONE FORMATION IN DENTAL IMPLANT
- MMP13-Overexpressing Mesenchymal Stem Cells Enhance Bone Tissue Formation in the Presence of Collagen Hydrogel