J Periodontal Implant Sci.
2013 Aug;43(4):168-176. 10.5051/jpis.2013.43.4.168.
The effects of dexamethasone on the apoptosis and osteogenic differentiation of human periodontal ligament cells
- Affiliations
-
- 1Department of Periodontology, Kyungpook National University School of Dentistry, Daegu, Korea. jysuh@knu.ac.kr
- 2Institute for Hard Tissue and Bio-Tooth Regeneration, Kyungpook National University School of Dentistry, Daegu, Korea.
- KMID: 2027812
- DOI: http://doi.org/10.5051/jpis.2013.43.4.168
Abstract
- PURPOSE
The purpose of the current study was to examine the effect of dexamethasone (Dex) at various concentrations on the apoptosis and mineralization of human periodontal ligament (hPDL) cells.
METHODS
hPDL cells were obtained from the mid-third of premolars extracted for orthodontic reasons, and a primary culture of hPDL cells was prepared using an explant technique. Groups of cells were divided according to the concentration of Dex (0, 1, 10, 100, and 1,000 nM). A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed for evaluation of cellular viability, and alkaline phosphatase activity was examined for osteogenic differentiation of hPDL cells. Alizarin Red S staining was performed for observation of mineralization, and real-time polymerase chain reaction was performed for the evaluation of related genes.
RESULTS
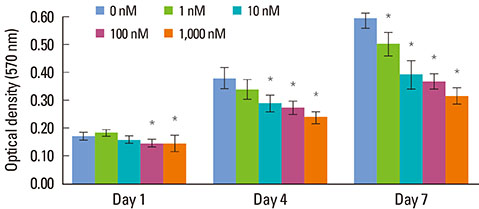
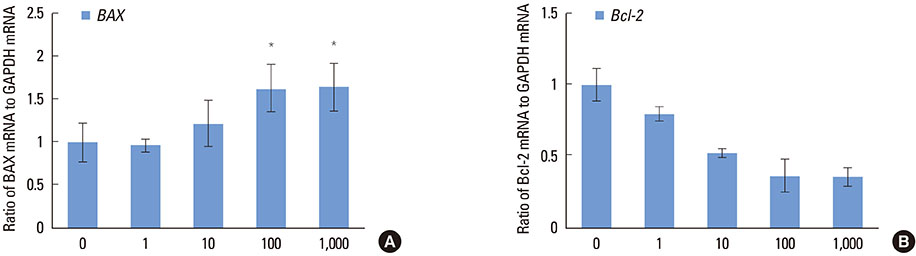
Increasing the Dex concentration was found to reduce cellular viability, with an increase in alkaline phosphatase activity and mineralization. Within the range of Dex concentrations tested in this study, 100 nM of Dex was found to promote the most vigorous differentiation and mineralization of hPDL cells. Dex-induced osteogenic differentiation and mineralization was accompanied by an increase in the level of osteogenic and apoptosis-related genes and a reduction in the level of antiapoptotic genes. The decrease in hPDL cellular viability by glucocorticoid may be explained in part by the increased prevalence of cell apoptosis, as demonstrated by BAX expression and decreased expression of the antiapoptotic gene, Bcl-2.
CONCLUSIONS
An increase in hPDL cell differentiation rather than cellular viability at an early stage is likely to be a key factor in glucocorticoid induced mineralization. In addition, apoptosis might play an important role in Dex-induced tissue regeneration; however, further study is needed for investigation of the precise mechanism.
MeSH Terms
Figure
Reference
-
1. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.
Article2. Bartold PM, Shi S, Gronthos S. Stem cells and periodontal regeneration. Periodontol 2000. 2006; 40:164–172.
Article3. Gould TR, Melcher AH, Brunette DM. Migration and division of progenitor cell populations in periodontal ligament after wounding. J Periodontal Res. 1980; 15:20–42.
Article4. McCulloch CA, Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res. 1991; 26(3 Pt 1):144–154.
Article5. Chung CH, Golub EE, Forbes E, Tokuoka T, Shapiro IM. Mechanism of action of beta-glycerophosphate on bone cell mineralization. Calcif Tissue Int. 1992; 51:305–311.
Article6. Kodama HA, Amagai Y, Sudo H. Culture conditions affecting differentiation and calcification in the MC3T3-E1 osteogenic cell line. In : Ali SY, editor. Cell-mediated calcification and matrix vesicles. Amsterdam: Elsevier Science BV;2006. p. 297–302.7. Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res. 1994; 9:843–854.
Article8. Majeska RJ, Nair BC, Rodan GA. Glucocorticoid regulation of alkaline phosphatase in the osteoblastic osteosarcoma cell line ROS 17/2.8. Endocrinology. 1985; 116:170–179.
Article9. Leboy PS, Beresford JN, Devlin C, Owen ME. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol. 1991; 146:370–378.
Article10. Shalhoub V, Conlon D, Tassinari M, Quinn C, Partridge N, Stein GS, et al. Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J Cell Biochem. 1992; 50:425–440.
Article11. Abe Y, Aida Y, Abe T, Hirofuji T, Anan H, Maeda K. Development of mineralized nodules in fetal rat mandibular osteogenic precursor cells: requirement for dexamethasone but not for beta-glycerophosphate. Calcif Tissue Int. 2000; 66:66–69.
Article12. Bellows CG, Heersche JN, Aubin JE. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev Biol. 1990; 140:132–138.
Article13. Wang H, Pang B, Li Y, Zhu D, Pang T, Liu Y. Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy. 2012; 14:423–430.
Article14. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998; 102:274–282.
Article15. Parfitt AM. Bone-forming cells in clinical conditions. In : Hall BK, editor. Bone. Caldwell: Telford Press and CRC Press;1990. p. 351–429.16. Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998; 13:793–802.
Article17. Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000; 87:1055–1062.18. Shin JH, Park JW, Yeo SI, Noh WC, Kim MK, Kim JC, et al. Identification of matrix mineralization-related genes in human periodontal ligament cells using cDNA microarray. J Korean Acad Periodontol. 2007; 37:Suppl. 447–463.
Article19. Kim HS, Park JW, Yeo SI, Choi BJ, Suh JY. Effects of high glucose on cellular activity of periodontal ligament cells in vitro. Diabetes Res Clin Pract. 2006; 74:41–47.
Article20. Bligh ME, Bhagwat SA, Castonguay TW. Aldosterone diurnal rhythm in the rat: a question of cross-reactivity? Physiol Behav. 1993; 53:845–848.
Article21. Malendowicz LK, Nussdorfer GG, Markowska A, Tortorella C, Nowak M, Warchol JB. Effects of neuromedin U (NMU)-8 on the rat hypothalamo-pituitary-adrenal axis. Evidence of a direct effect of NMU-8 on the adrenal gland. Neuropeptides. 1994; 26:47–53.
Article22. Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF. A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology. 1995; 136:5336–5342.
Article23. Sharma AC, Bosmann HB, Motew SJ, Hales KH, Hales DB, Ferguson JL. Steroid hormone alterations following induction of chronic intraperitoneal sepsis in male rats. Shock. 1996; 6:150–154.
Article24. Ishida Y, Bellows CG, Tertinegg I, Heersche JN. Progesterone-mediated stimulation of osteoprogenitor proliferation and differentiation in cell populations derived from adult or fetal rat bone tissue depends on the serum component of the culture media. Osteoporos Int. 1997; 7:323–330.
Article25. Tenenbaum HC, Heersche JN. Differentiation of osteoblasts and formation of mineralized bone in vitro. Calcif Tissue Int. 1982; 34:76–79.
Article26. Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002; 966:73–81.
Article27. Beloti MM, Rosa AL. Osteoblast differentiation of human bone marrow cells under continuous and discontinuous treatment with dexamethasone. Braz Dent J. 2005; 16:156–161.
Article28. McCulloch CA, Tenenbaum HC. Dexamethasone induces proliferation and terminal differentiation of osteogenic cells in tissue culture. Anat Rec. 1986; 215:397–402.
Article29. Shen Q, Zhu S, Hu J, Geng N, Zou S. Recombinant human bone morphogenetic protein-4 (BMP-4)-stimulated cell differentiation and bone formation within the expanding calvarial suture in rats. J Craniofac Surg. 2009; 20:1561–1565.
Article30. Choi MH, Noh WC, Park JW, Lee JM, Suh JY. Gene expression pattern during osteogenic differentiation of human periodontal ligament cells in vitro. J Periodontal Implant Sci. 2011; 41:167–175.
Article31. Cheng SL, Zhang SF, Avioli LV. Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. J Cell Biochem. 1996; 61:182–193.
Article32. Hayami T, Zhang Q, Kapila Y, Kapila S. Dexamethasone's enhancement of osteoblastic markers in human periodontal ligament cells is associated with inhibition of collagenase expression. Bone. 2007; 40:93–104.
Article33. Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990; 4:3111–3123.
Article34. Porter RM, Huckle WR, Goldstein AS. Effect of dexamethasone withdrawal on osteoblastic differentiation of bone marrow stromal cells. J Cell Biochem. 2003; 90:13–22.
Article35. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002; 108:17–29.
Article36. Lynch MP, Capparelli C, Stein JL, Stein GS, Lian JB. Apoptosis during bone-like tissue development in vitro. J Cell Biochem. 1998; 68:31–49.
Article37. Ducy P, Geoffroy V, Karsenty G. Study of osteoblast-specific expression of one mouse osteocalcin gene: characterization of the factor binding to OSE2. Connect Tissue Res. 1996; 35:7–14.
Article38. Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW. Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta. 2006; 1762:191–201.
Article39. Seo MS, Hwang KG, Kim H, Baek SH. Analysis of gene expression during odontogenic differentiation of cultured human dental pulp cells. Restor Dent Endod. 2012; 37:142–148.
Article40. Fujita H, Yamamoto M, Ogino T, Kobuchi H, Ohmoto N, Aoyama E, et al. Necrotic and apoptotic cells serve as nuclei for calcification on osteoblastic differentiation of human mesenchymal stem cells in vitro. Cell Biochem Funct. 2013; 05. 08. [Epub]. http://dx.doi.org/doi:10.1002/cbf.2974.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of nitric oxide on the proliferation and differentiation of human periodontal ligament cells
- Analysis of gene expression during mineralization of cultured human periodontal ligament cells
- Valproic Acid Modulates the Multipotency in Periodontal Ligament Stem Cells via p53-Mediated Cell Cycle
- Indirect Co-Culture of Stem Cells from Human Exfoliated Deciduous Teeth and Oral Cells in a Microfluidic Platform
- Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars