J Korean Soc Magn Reson Med.
2014 Sep;18(3):244-252. 10.13104/jksmrm.2014.18.3.244.
Cardiac Magnetic Resonance Imaging Using Multi-physiological Intelligent Trigger System
- Affiliations
-
- 1Department of Electrical Engineering, Kwangwoon University, Seoul, Korea. cbahn@kw.ac.kr
- KMID: 1999924
- DOI: http://doi.org/10.13104/jksmrm.2014.18.3.244
Abstract
- PURPOSE
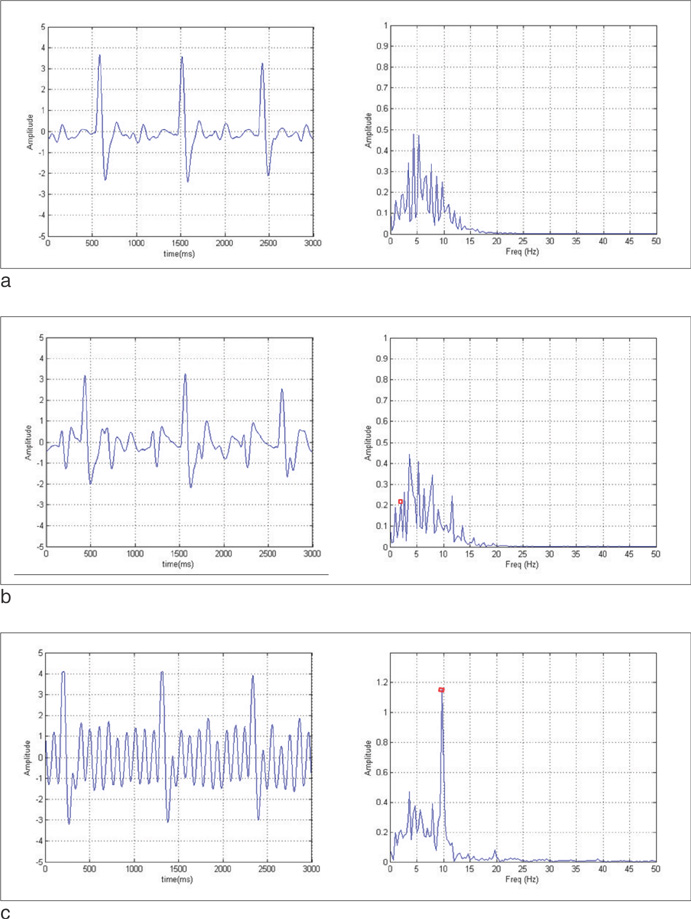
We proposed a multi-physiological signals based real-time intelligent triggering system(MITS) for Cardiac MRI. Induced noise of the system was analyzed.
MATERIALS AND METHODS
MITS makes cardiac MR imaging sequence synchronize to the cardiac motion using ECG, respiratory signal and second order derivative of SPO2 signal. Abnormal peaks due to arrhythmia or subject's motion are rejected using the average R-R intervals and R-peak values. Induced eddy currents by gradients switching in cardiac MR imaging are analyzed. The induced eddy currents were removed by hardware and software filters.
RESULTS
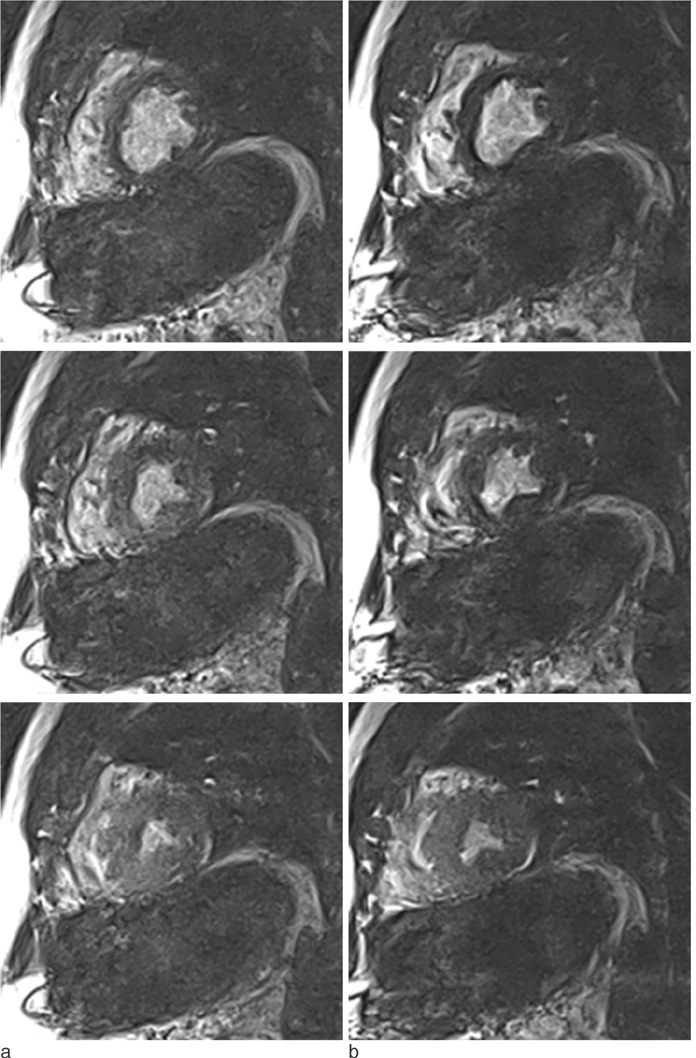
Cardiac MR images that synchronized to the cardiac and respiratory motion are acquired using MITS successfully without artifacts caused by induced eddy currents of gradient switching or subject's motion or arrhythmia. We showed that the second order derivative of the SPO2 signal can be used as a complement to the ECG signals.
CONCLUSION
The proposed system performs cardiac and respiratory gating with multi-physiological signals in real time. During the cardiac gating, induced noise caused by eddy currents is removed. False triggers due to subject's motion or arrhythmia are rejected. The cardiac MR imaging with free breathing is obtained using MITS.
Keyword
MeSH Terms
Figure
Reference
-
1. Vivian S. Cardiovascular MRI: Physical Principles to Practical Protocols. Philadelphia: Lippincott Williams & Wilkins;2006. p. 257–265.2. Kaus MR, Berg JV, Weese J, Niessen W, Pekar V. Automated segmentation of the left ventricle in cardiac MRI. Med Image Anal. 2004; 8:245–254.3. Amparo EG, Higgins CB, Farmer D, Gamsu G, McNamara M. Gated MRI of cardiac and paracardiac masses: initial experience. AJR Am J Roentgenol. 1984; 143:1151–1156.4. Yang YJ, Park J, Hong HJ, Ahn CB. Multi-biological Signal-based Smart Trigger System for Cardiac MRI. Trans KIEE. 2014; to be published.5. Moghari MH, Roujol S, Chan RH, et al. Free-breathing 3D cardiac MRI using iterative image-based respiratory motion correction. Magn Reson Med. 2013; 70:1005–1015.6. Pang J, Bhat H, Sharif B, et al. Whole-heart coronary MRA with 100% respiratory gating efficiency: Self-navigated three-dimensional retrospective image-based motion correction (TRIM). Magn Reson Med. 2014; 71:67–74.7. Vaninbroukx J, Bosmans H, Sunaert S, et al. The use of ECG and respiratory triggering to improve the sensitivity of oxygen-enhanced proton MRI of lung ventilation. Eur Radiol. 2003; 13:1260–1265.8. Li XA, Stepaniak C, Gore E. Technical and dosimetric aspects of respiratory gating using a pressure-sensor motion monitoring system. Med Phys. 2006; 33:145–154.9. Kandpal H, Sharma R, Madhusudhan KS, Kapoor KS. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and apparent diffusion coefficient values. AJR Am J Roentgenol. 2009; 192:915–922.10. Udupa JK, Murthy IS. Syntactic approach to ECG rhythm analysis. IEEE Trans Biomed Eng. 1980; 27:370–375.11. Chang KM. Arrhythmia ECG noise reduction by ensemble empirical mode decomposition. Sensors. 2010; 10:6063–6080.12. Wiesmann F, Szimtenings M, Frydrychowicz A, et al. High-resolution MRI with cardiac and respiratory gating allows for accurate in vivo atherosclerotic plaque visualization in the murine aortic arch. Magn Reson Med. 2003; 50:69–74.13. Laudon MK, Webster JG, Frayne R, Grist TM. Minimizing interference from magnetic resonance imagers during electrocardiography. IEEE Trans Biomed Eng. 1998; 45:160–164.14. Ahn CB, Kim PK. Method and apparatus for detecting feature points using distribution of feature points in second derivative of photoplethysmogram waveform. Korean Patent. 10-1036233. 2011.15. Worthley SG, Helft G, Fayad ZA, et al. Cardiac gated breath-hold black blood MRI of the coronary artery wall: an in vivo and ex vivo comparison. Int J Cardiovasc Imaging. 2001; 17:195–201.16. Hofman MB, Paschal CB, Li D, Haacke EM, van Rossum AC, Sprenger M. MRI of coronary arteries: 2D breath-hold vs 3D respiratory-gated acquisition. J Comput Assist Tomogr. 1995; 19:56–62.17. Ulvi H, Yoldas¸ T, Yiğiter R, Müngen B. R-R interval variation and the sympathetic skin response in the assessment of the autonomic nervous system in leprosy patients. Acta Neurol Scand. 2003; 107:42–49.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stress-Induced Cardiomyopathy: Assessment with Cardiac Magnetic Resonance Imaging and Multi-Detector Computed Tomography
- High field strength magnetic resonance imaging of cardiovascular diseases
- Treatment Response Evaluation of Cardiac Amyloidosis Using Serial T1- and T2-Mapping Cardiovascular Magnetic Resonance Imaging
- Efforts of the Past 20 Years for Proved Magnetic Resonance Imaging Safety of Medtronic Implantable Cardiac Devices
- ECG-Gated NMR Imaging of a Ventricular Septal Defect