Early Experience With Active Surveillance in Low-Risk Prostate Cancer Treated
- Affiliations
-
- 1Department of Urology, Keimyung University School of Medicine, Daegu, Korea. cikim@dsmc.or.kr
- KMID: 1988419
- DOI: http://doi.org/10.4111/kju.2014.55.3.167
Abstract
- PURPOSE
This study was conducted to describe our early experience with active surveillance (AS).
MATERIALS AND METHODS
Between January 2008 and December 2012, 35 patients were treated with AS. Selection criteria included the following: Gleason score < or =6 with single positive core, clinical stage < or =T1c, prostate-specific antigen (PSA) < or =10 ng/mL, and unremarkable imaging results. On patient follow-up, we regularly measured PSA (every 3-6 months) and performed prostate biopsies (after 1 and 3 years).
RESULTS
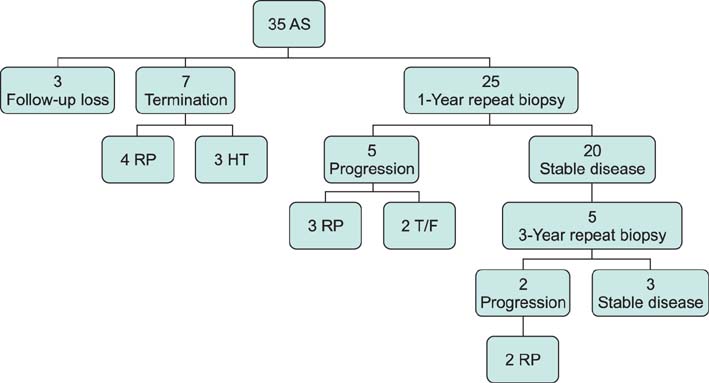
In the first year of follow-up, prostate biopsies were performed in 25 patients (13 patients, negative for cancer; 7 patients, Gleason score of 6 without progression; 5 patients, progression, treated with radical prostatectomy [RP]). In the third year of follow-up, prostate biopsies were performed in five patients (two patients, negative for cancer; one patient, Gleason score of 6 without progression; two patients, progression, treated with RP). Seven patients discontinued AS because of increased anxiety, and three patients were lost to follow-up. Overall, seven patients (28%) who experienced progression had a mean PSA doubling time (DT) of 7.54 years. Six patients had a PSA DT of more than 3 years, whereas one had a PSA DT of less than 3 years. This study was limited by its small sample size and short follow-up period.
CONCLUSIONS
PSA kinetics did not correlate with progression, which suggests that regular biopsies should still be performed. AS is an available treatment option for patients with a low risk of prostate cancer but should only be used in carefully selected patients.
MeSH Terms
Figure
Cited by 3 articles
-
Current role of multiparametric magnetic resonance imaging in the management of prostate cancer
Nikolas Christopher Katelaris, Damien Michael Bolton, Mahesha Weerakoon, Liam Toner, Phillip Mark Katelaris, Nathan Lawrentschuk
Korean J Urol. 2015;56(5):337-345. doi: 10.4111/kju.2015.56.5.337.Updated clinical results of active surveillance of very-low-risk prostate cancer in Korean men: 8 years of follow-up
Ji Yong Ha, Teak Jun Shin, Wonho Jung, Byung Hoon Kim, Choal Hee Park, Chun Il Kim
Investig Clin Urol. 2017;58(3):164-170. doi: 10.4111/icu.2017.58.3.164.Efficacy and Safety of Robotic Procedures Performed Using the da Vinci Robotic Surgical System at a Single Institute in Korea: Experience with 10000 Cases
Dong Hoon Koh, Won Sik Jang, Jae Won Park, Won Sik Ham, Woong Kyu Han, Koon Ho Rha, Young Deuk Choi
Yonsei Med J. 2018;59(8):975-981. doi: 10.3349/ymj.2018.59.8.975.
Reference
-
1. Park SK, Sakoda LC, Kang D, Chokkalingam AP, Lee E, Shin HR, et al. Rising prostate cancer rates in South Korea. Prostate. 2006; 66:1285–1291.2. The statistics report: the incidence of cancer on 1999-2011 and the survival rate on 2012 [Internet]. Goyang: National Cancer Center;cited 2012 Dec 1. Available from: http://www.ncc.re.kr.3. Carter HB, Walsh PC, Landis P, Epstein JI. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002; 167:1231–1234.4. Choo R, Klotz L, Danjoux C, Morton GC, DeBoer G, Szumacher E, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002; 167:1664–1669.5. Klotz L. Active surveillance for favorable-risk prostate cancer: who, how and why? Nat Clin Pract Oncol. 2007; 4:692–698.6. Lawrentschuk N, Klotz L. Active surveillance for favorable-risk prostate cancer: a short review. Korean J Urol. 2010; 51:665–670.7. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010; 28:126–131.8. van den Bergh RC, Roemeling S, Roobol MJ, Aus G, Hugosson J, Rannikko AS, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol. 2009; 55:1–8.9. Kakehi Y, Kamoto T, Shiraishi T, Ogawa O, Suzukamo Y, Fukuhara S, et al. Prospective evaluation of selection criteria for active surveillance in Japanese patients with stage T1cN0M0 prostate cancer. Jpn J Clin Oncol. 2008; 38:122–128.10. Dall'Era MA, Konety BR, Cowan JE, Shinohara K, Stauf F, Cooperberg MR, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008; 112:2664–2670.11. Berglund RK, Masterson TA, Vora KC, Eggener SE, Eastham JA, Guillonneau BD. athological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008; 180:1964–1967.12. Soloway MS, Soloway CT, Williams S, Ayyathurai R, Kava B, Manoharan M. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008; 101:165–169.13. Reynard J, Brewster S, Biers S. Oxford handbook of urology. PSA derivatives and kinetics: free-to-total, density, velocity, and doubling time. Oxford: Oxford University Press;2010.14. Fowler FJ Jr, McNaughton Collins M, Albertsen PC, Zietman A, Elliott DB, Barry MJ. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000; 283:3217–3222.15. Barocas DA, Cowan JE, Smith JA Jr, Carroll PR. CaPSURE Investigators. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J Urol. 2008; 180:1330–1334.16. Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994; 271:368–374.17. Bastian PJ, Mangold LA, Epstein JI, Partin AW. Characteristics of insignificant clinical T1c prostate tumors: a contemporary analysis. Cancer. 2004; 101:2001–2005.18. Dall'Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012; 62:976–983.19. Fradet V, Kurhanewicz J, Cowan JE, Karl A, Coakley FV, Shinohara K, et al. Prostate cancer managed with active surveillance: role of anatomic MR imaging and MR spectroscopic imaging. Radiology. 2010; 256:176–183.20. Afaq A, Koh DM, Padhani A, van As N, Sohaib SA. Clinical utility of diffusion-weighted magnetic resonance imaging in prostate cancer. BJU Int. 2011; 108:1716–1722.21. Ross AE, Loeb S, Landis P, Partin AW, Epstein JI, Kettermann A, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010; 28:2810–2816.22. Whitson JM, Porten SP, Hilton JF, Cowan JE, Perez N, Cooperberg MR, et al. The relationship between prostate specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J Urol. 2011; 185:1656–1660.23. Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011; 29:2185–2190.24. van As NJ, Norman AR, Thomas K, Khoo VS, Thompson A, Huddart RA, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008; 54:1297–1305.25. Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011; 29:3669–3676.26. Chung JS, Han BK, Jeong SJ, Hong SK, Byun SS, Choe G, et al. Pathologic outcome of unilateral low risk prostate cancers on multicore prostate biopsy after radical prostatectomy. Korean J Urol. 2008; 49:874–878.27. Lee SE, Kim DS, Lee WK, Park HZ, Lee CJ, Doo SH, et al. Application of the Epstein criteria for prediction of clinically insignificant prostate cancer in Korean men. BJU Int. 2010; 105:1526–1530.28. Kim SC, Hong JH, Song K, Jeong IG, Song C, Kim CS, et al. Predictive factors for upgrading or upstaging in biopsy gleason score 6 prostate cancer. Korean J Urol. 2009; 50:836–842.29. Ahn HJ, Ko YH, Jang HA, Kang SG, Kang SH, Park HS, et al. Single positive core prostate cancer in a 12-core transrectal biopsy scheme: clinicopathological implications compared with multifocal counterpart. Korean J Urol. 2010; 51:671–676.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiparametric MRI in Active Surveillance of Prostate Cancer: An Overview and a Practical Approach
- Active Surveillance for Favorable-Risk Prostate Cancer: A Short Review
- Impact of family history of prostate cancer on disease progression for prostatic cancer patients undergoing active surveillance: A systematic review and meta-analysis
- Outcomes of Active Surveillance in Localized Prostate Cancer
- A Review of Active Surveillance of Papillary Thyroid Microcarcinoma