J Korean Acad Conserv Dent.
2002 Nov;27(6):587-599. 10.5395/JKACD.2002.27.6.587.
Response characteristics of ventral posteromedial thalamic nociceptive neurons in the anesthetized rat
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Chonbuk National University, Korea.
- 2Department of Oral Physiology and Institute of Oral Bioscience, College of Dentistry, Chonbuk National University, Korea.
- KMID: 1987317
- DOI: http://doi.org/10.5395/JKACD.2002.27.6.587
Abstract
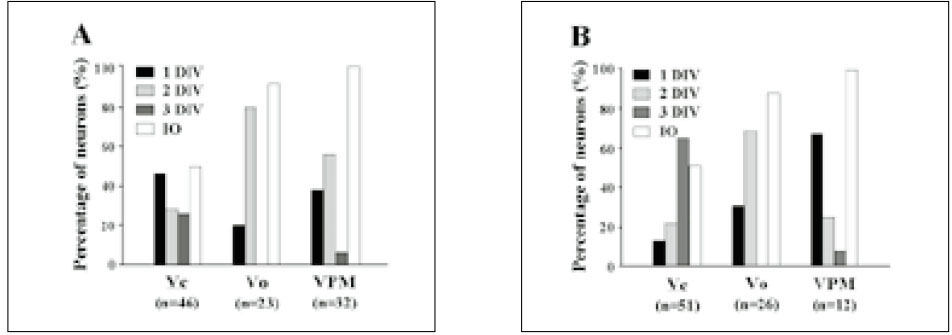
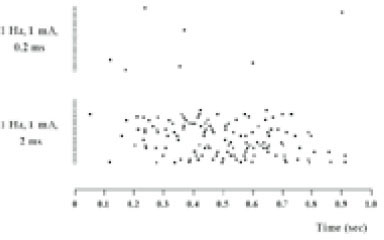
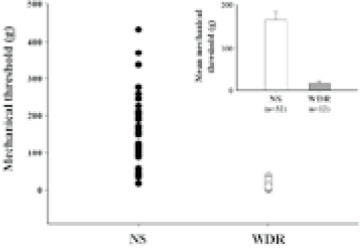
- Extracellular single unit recordings were made from the ventral posteromedial thalamic (VPM) nociceptive neurons to determine mechanoreceptive field (RF) and response properties. A total of 44 VPM thalamic nociceptive neurons were isolated from rats anesthetized with urethane-chloralose. Based on responses to various mechanical stimuli including touch, pressure and pinch applied to the RF, 32 of 44 neurons were classified as nociceptive specific (NS) neuron. The other 12 neurons, classified as wide dynamic range (WDR), showed a graded response to increasingly intense stimuli, with a maximum discharge to noxious pinch. The VPM nociceptive neurons showed various spontaneous activity ranged from 0-6 Hz. They were located throughout the VPM, and had an contralateral RF including mainly intraoral (and perioral) regions. The RF size was relatively small, and very few neurons had a receptive field involving 3 trigeminal divisions. The NS neurons activated only by pressure and pinch stimuli had high mechanical thresholds compared to WDR neurons activated also by touch stimuli. The VPM nociceptive neurons were tested with suprathershold graded mechanical stimuli. Most of 21 NS and 8 WDR neurons showed a progressive increase in number of spikes as mechanical stimulus intensity was increased. In some neurons, the responses reached a peak before the highest intensity was given. Application of 5 mM CoCl2 (10 microl) solution to the trigeminal subnucleus caudalis did not produce any significant changes in the spontaneous activity, RF size, mechanical threshold, and response to suprathresold mechanical stimuli of 9 VPM nociceptive neurons tested. 17 of 33 VPM nociceptive neurons responded to noxious heat as well as noxious mechanical stimuli applied to their RF. Application of the mustard oil, a small-fiber excitant and inflammatory irritant, to the right maxillary first molar tooth pulp induced an immediate but short-lasting neuronal discharges upto approximately 4 min in 16 of 42 VPM nociceptive neurons. These results suggest that VPM thalamic nucleus may contribute to the sensory discriminative aspect of orofacial nociception.
Keyword
MeSH Terms
Figure
Reference
-
1. Albe-Fessard D, Berkley KJ, Kruger L, Ralston HJ, Willis WD. Diencephalic mechanisms of pain sensation. Brain Res. 1985. 356:217–296.2. Craig AD, Dostrovsky JO. Wall PD, Melzack R, editors. Medulla to Thalamus. Textbook of Pain. 1999. Edinburgh: Churchill-Livingstone;183–214.3. Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000. 11:57–91.
Article4. Apkarian AV, Hodge CJ. Primate spinothalamic pathways: III. Thalamic terminations of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol. 1989. 288:493–511.
Article5. Craig AD, Linington AJ, Kniffki KD. Cells of origin of spinothalamic tract projections to the medial and lateral thalamus in the cat. J Comp Neurol. 1989. 289:568–585.
Article6. Ganchrow D. Intratrigeminal and thalamic projections of nucleus caudalis in the squirrel monkey (Saimiri sciureus): a degeneration and autoradiographic study. J Comp Neurol. 1978. 178:281–312.
Article7. Casey KL, Morrow TJ. Ventral posterior thalamic neurons differentially responsive to noxious stimulation of the awake monkey. Science. 1983. 221:675–677.
Article8. Chung JM, Lee KH, Surmeier DJ, Sorkin LS, Kim J, Willis WD. Response characteristics of neurons in the ventral posterior lateral nucleus of the monkey thalamus. J Neurophysiol. 1986. 56:370–390.
Article9. Kenshalo DR, Giesler GJ, Leonard RB, Willis WD. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol. 1980. 43:1594–1614.
Article10. Brinkhus HB, Carstens E, Zimmermann M. Encoding of graded noxious skin heating by neurons in posterior thalamus and adjacent areas in the cat. Neurosci Lett. 1979. 15:37–42.
Article11. Honda CN, Mense S, Perl ER. Neurons in ventrobasal region of cat thalamus selectively responsive to noxious mechanical stimulation. J Neurophysiol. 1983. 49:662–673.
Article12. Yokota T, Asato F, Koyama N, Masuda T, Taguchi H. Nociceptive body representation in nucleus ventralis posterolateralis of cat thalamus. J Neurophysiol. 1988. 60:1714–1727.
Article13. Simone DA, Hanson ME, Bernau NA, Pubols BH. Nociceptive neurons of the raccoon lateral thalamus. J Neurophysiol. 1993. 69:318–328.
Article14. Benoist JM, Kayser V, Gautron M, Guilbaud G. Low dose of morphine strongly depresses responses of specific nociceptive neurones in the ventrobasal complex of the rat. Pain. 1983. 15:333–344.
Article15. Guilbaud G, Peschanski M, Gautron M, Binder D. Neurones responding to noxious stimulation in VB complex and caudal adjacent regions in the thalamus of the rat. Pain. 1980. 8:303–318.
Article16. Mitchell D, Hellon RF. Neuronal and behavioural responses in rats during noxious stimulation of the tail. Proc R Soc Lond B Biol Sci. 1977. 197:169–194.
Article17. Peschanski M, Guilbaud G, Gautron M, Besson JM. Encoding of noxious heat messages in neurons of the ventrobasal thalamic complex of the rat. Brain Res. 1980. 197:401–413.
Article18. Yokota T, Matsumoto N. Location and functional organization of trigeminal wide dynamic range neurons within the nucleus ventralis posteromedialis of the cat. Neurosci Lett. 1983. 39:231–236.
Article19. Yokota T, Matsumoto N. Somatotopic distribution of trigeminal nociceptive specific neurons within the caudal somatosensory thalamus of cat. Neurosci Lett. 1983. 39:125–130.
Article20. Yokota T, Koyama N, Matsumoto N. Somatotopic distribution of trigeminal nociceptive neurons in ventrobasal complex of cat thalamus. J Neurophysiol. 1985. 53:1387–1400.
Article21. Bushnell MC, Duncan GH. Mechanical response properties of ventroposterior medial thalamic neurons in the alert monkey. Exp Brain Res. 1987. 67:603–614.
Article22. Iwata K, Kenshalo DR, Dubner R, Nahin RL. Diencephalic projections from the superficial and deep laminae of the medullary dorsal horn in the rat. J Comp Neurol. 1992. 321:404–420.
Article23. Peschanski M. Trigeminal afferents to the diencephalon in the rat. Neuroscience. 1984. 12:465–487.
Article24. Barnett EM, Evans GD, Sun N, Perlman S, Cassell MD. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci. 1995. 15:2972–2984.
Article25. Hu JW. Response properties of nociceptive and nonnociceptive neurons in the rat's trigeminal subnucleus caudalis (medullary dorsal horn) related to cutaneous and deep craniofacial afferent stimulation and modulation by diffuse noxious inhibitory controls. Pain. 1990. 41:331–345.
Article26. Vahle-Hinz C, Gottschaldt KM. Macchi G, Rustiont A, Spreafico R. Principal differences in the organization of the thalamic face representation in rodents and felids. Somatosensory integration in the thalamus. 1983. Amsterdam: Elsevier Science;125–145.27. Vos BP, Benoist JM, Gautron M, Guilbaud G. Changes in neuronal activities in the two ventral posterior medial thalamic nuclei in an experimental model of trigeminal pain in the rat by constriction of one infraorbital nerve. Somatosens Mot Res. 2000. 17:109–122.
Article28. Dallel R, Raboisson P, Auroy P, Woda A. The rostral part of the trigeminal sensory complex is involved in orofacial nociception. Brain Res. 1988. 448:7–19.
Article29. Shigenaga Y, Matano S, Okada K, Sakai A. The effects of tooth pulp stimulation in the thalamus and hypothalamus of the rat. Brain Res. 1973. 63:402–407.
Article30. Yokota T, Nishikawa Y, Koyama N. Tooth pulp input to the shell region of nucleus ventralis posteromedialis of the cat thalamus. J Neurophysiol. 1986. 56:80–98.
Article31. Allen GV, Pronych SP. Trigeminal autonomic pathways involved in nociception-induced reflex cardiovascular responses. Brain Res. 1997. 754:269–278.
Article32. Hochstenbach SL, Ciriello J. Medullary pathways mediating depressor responses from Na+-sensitive sites in nucleus of the solitary tract. Am J Physiol. 1997. 272:R126–R133.
Article33. Lee C, Malpeli JG. Somata-selective lesions induced by cobaltous chloride: a parametric study. Brain Res. 1986. 364:396–399.
Article34. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1998. New York: Academic.35. Dallel R, Raboisson P, Woda A, Sessle BJ. Properties of nociceptive and non-nociceptive neurons in trigeminal subnucleus oralis of the rat. Brain Res. 1990. 521:95–106.
Article36. Park SJ, Chiang CY, Hu JW, Sessle BJ. Neuroplasticity induced by tooth pulp stimulation in trigeminal subnucleus oralis involves NMDA receptor mechanisms. J Neurophysiol. 2001. 85:1836–1846.
Article37. Cliffer KD, Burstein R, Giesler GJ. Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci. 1991. 11:852–868.
Article38. Willis WD. Steriade M, Jones EG, McCormick DA, editors. Nociceptive functions of thalamic neurons. Thalamus, experimental and clinical aspects, Vol. II. 1997. Amsterdam: Elsevier Science;373–424.39. Gingold SI, Greenspan JD, Apkarian AV. Anatomic evidence of nociceptive inputs to primary somatosensory cortex: relationship between spinothalamic terminals and thalamocortical cells in squirrel monkeys. J Comp Neurol. 1991. 308:467–490.
Article40. Jones EG, Friedman DP. Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. J Neurophysiol. 1982. 48:521–544.
Article41. Bushnell MC, Duncan GH, Tremblay N. Thalamic VPM nucleus in the behaving monkey I Multimodal and discriminative properties of thermosensitive neurons. J Neurophysiol. 1993. 69:739–752.
Article42. Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999. 81:2243–2252.
Article43. Kniffki KD, vahle-Hinz C. Besson JM, Guilbaud G, Peschanski M. The periphery of the cat's ventroposteromedial nucleus (VPMp): nociceptive neurones. Thalamus and Pain. 1987. Amsterdam: Elsevier;245–257.44. Emmers R. Stimulation of the periaqueductal gray subdues sensitized pain in morphine- and meperidinedependent rats. Exp Neurol. 1985. 88:405–417.
Article45. Yoshida A, Dostrovsky JO, Sessle BJ, Chiang CY. Trigeminal projections to the nucleus submedius of the thalamus in the rat. J Comp Neurol. 1991. 307:609–625.
Article46. Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966. 16:316–332.
Article47. Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996. 66:105–108.
Article48. Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999. 79:75–82.
Article49. Georgopoulos AP. Stimulus-response relations in high-threshold mechanothermal fibers innervating primate glabrous skin. Brain Res. 1977. 128:547–552.
Article50. Gybels J, Handwerker HO, Van Hees J. A comparison between the discharges of human nociceptive nerve fibres and the subject's ratings of his sensations. J Physiol. 1979. 292:193–206.
Article51. Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ. NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol. 1998. 80:2621–2631.
Article52. Naftel JP, Bernanke JM, Qian XB. Quantitative study of the apical nerve fibers of adult and juvenile rat molars. Anat Rec. 1994. 238:507–516.
Article53. Giesler GJ, Nahin RL, Madsen AM. Postsynaptic dorsal column pathway of the rat. I. Anatomical studies. J Neurophysiol. 1984. 51:260–275.
Article54. Cervero F, Iggo A, Molony V. Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol. 1977. 267:537–558.
Article55. Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol. 1996. 76:2661–2674.
Article56. Fukushima T, Kerr FW. Organization of trigeminothalamic tracts and other thalamic afferent systems of the brainstem in the rat: presence of gelatinosa neurons with thalamic connections. J Comp Neurol. 1979. 183:169–184.
Article57. Mantle-St John LA, Tracey DJ. Somatosensory nuclei in the brainstem of the rat: independent projections to the thalamus and cerebellum. J Comp Neurol. 1987. 255:259–271.
Article58. Bruce LL, McHaffie JG, Stein BE. The organization of trigeminotectal and trigeminothalamic neurons in rodents: a double-labeling study with fluorescent dyes. J Comp Neurol. 1987. 262:315–330.
Article59. Hagiwara S, Byerly L. Calcium channel. Annu Rev Neurosci. 1981. 4:69–125.
Article60. Marfurt CF, Turner DF. The central projections of tooth pulp afferent neurons in the rat as determined by the transganglionic transport of horseradish peroxidase. J Comp Neurol. 1984. 223:535–547.
Article61. Broton JG, Rosenfeld JP. Effects of trigeminal tractotomy on facial thermal nociception in the rat. Brain Res. 1985. 333:63–72.
Article62. Ikegami S, Kawamura H. Avoidance conditioning to tooth pulp stimulation in the cat after bulbar transection. Physiol Behav. 1979. 23:593–596.
Article63. Young RF. Effect of trigeminal tractotomy on dental sensation in humans. J Neurosurg. 1982. 56:812–818.
Article64. Young RF, Oleson TD, Perryman KM. Effect of trigeminal tractotomy on behavioral response to dental pulp stimulation in the monkey. J Neurosurg. 1981. 55:420–423.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distinct Role of Parvalbumin Expressing Neurons in the Reticular Thalamic Nucleus in Nociception
- Immunocytochemical Localization of Transforming Growth Factor-alpha in the Forebrain of the Rat
- The role of somatostatin in nociceptive processing of the spinal cord in anesthetized cats
- Distribution of Brain-Derived Neurotrophic Factor-Immunoreactive Neurons and Axon Terminals in the Rat Brain
- Ginsenosides Have a Suppressive Effect on c-Fos Expression in Brain and Reduce Cardiovascular Responses Increased by Noxious Stimulation to the Rat Tooth