J Korean Acad Conserv Dent.
2008 Jan;33(1):9-19. 10.5395/JKACD.2008.33.1.009.
The nanoleakage patterns of experimental hydrophobic adhesives after load cycling
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Seoul National University, Korea. hhson@snu.ac.kr
- 2Department of Conservative Dentistry, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea.
- KMID: 1986745
- DOI: http://doi.org/10.5395/JKACD.2008.33.1.009
Abstract
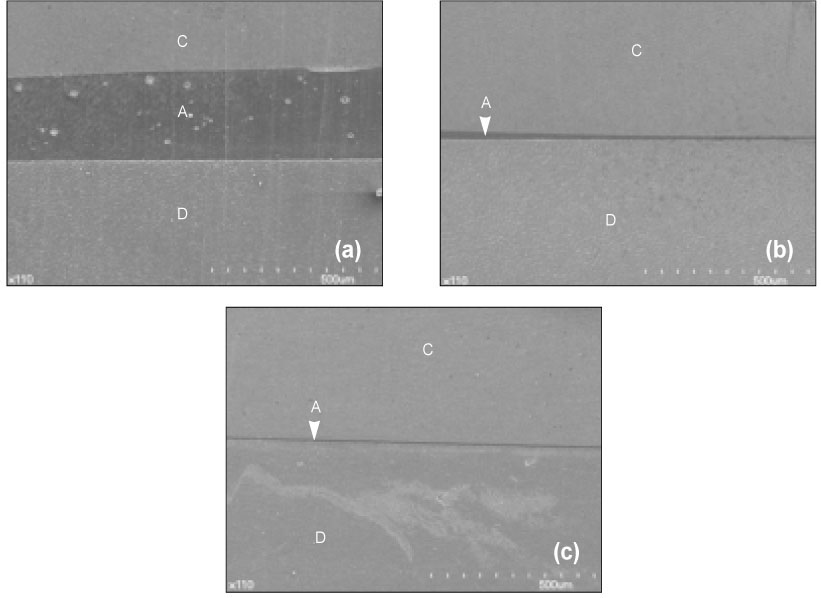
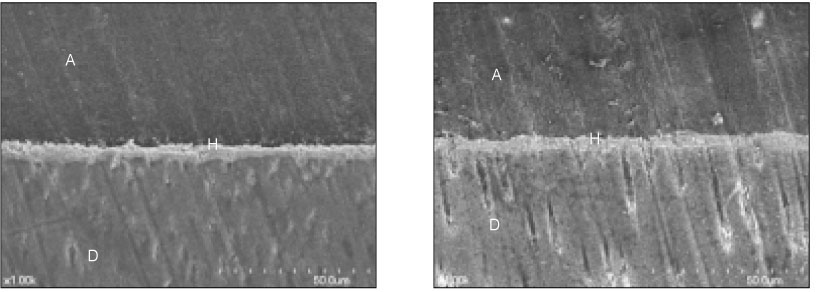
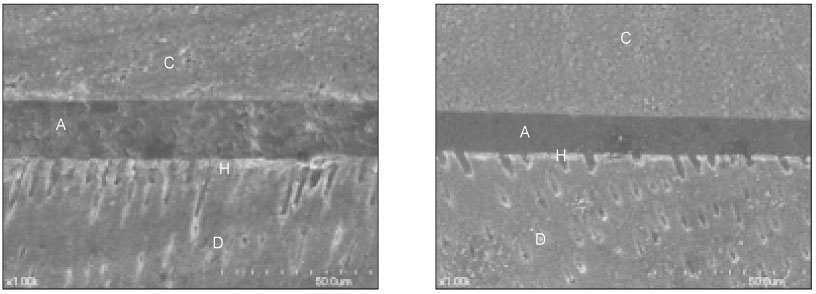
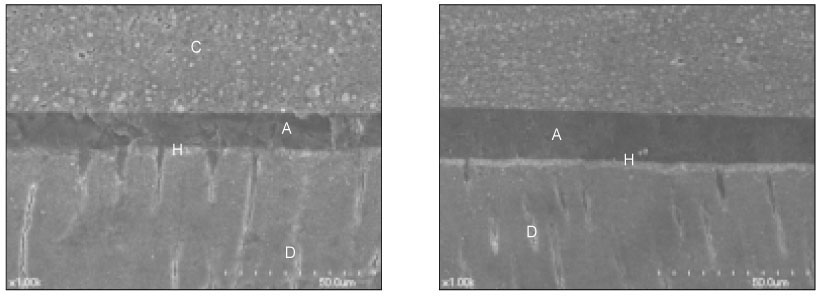
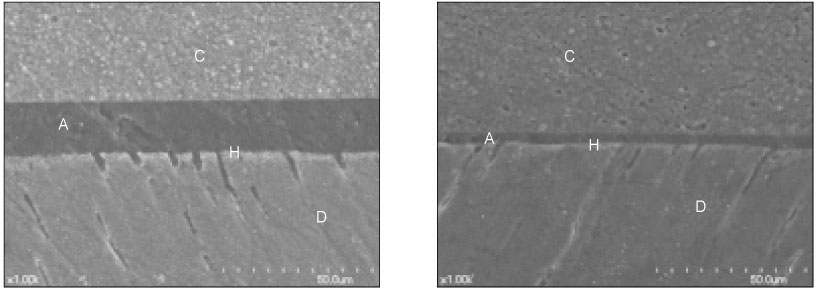
- The purpose of this study was: (1) to compare nanoleakage patterns of a conventional 3-step etch and rinse adhesive system and two experimental hydrophobic adhesive systems and (2) to investigate the change of the nanoleakage patterns after load cycling. Two kinds of hydrophobic experimental adhesives, ethanol containing adhesive (EA) and methanol containing adhesive (MA), were prepared. Thirty extracted human molars were embedded in resin blocks and occlusal thirds of the crowns were removed. The polished dentin surfaces were etched with a 35% phosphoric acid etching gel and rinsed with water. Scotchbond Multi-Purpose (MP), EA and MA were used for bonding procedure. Z-250 composite resin was built-up on the adhesive-treated surfaces. Five teeth of each dentin adhesive group were subjected to mechanical load cycling. The teeth were sectioned into 2 mm thick slabs and then stained with 50% ammoniacal silver nitrate. Ten specimens for each group were examined under scanning electron microscope in backscattering electron mode. All photographs were analyzed using image analysis software. Three regions of each specimen were used for evaluation of the silver uptake within the hybrid layer. The area of silver deposition was calculated and expressed in gray value. Data were statistically analyzed by two-way ANOVA and post-hoc testing of multiple comparisons was done with the Scheffe's test. Silver particles were observed in all the groups. However, silver particles were more sparsely distributed in the EA group and the MA group than in the MP group (p < .0001). There were no changes in nanoleakage patterns after load cycling.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The effect of the removal of chondroitin sulfate on bond strength of dentin adhesives and collagen architecture
Jong-Ryul Kim, Sang-Jin Park, Gi-Woon Choi, Kyoung-Kyu Choi
J Korean Acad Conserv Dent. 2010;35(3):211-221. doi: 10.5395/JKACD.2010.35.3.211.
Reference
-
1. Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955. 34:849–853.
Article2. Wang Y, Spencer P. Evaluation of the interface between one-bottle adhesive systems and dentin by Goldner's trichrome. Am J Dent. 2005. 18:66–72.3. Spencer P, Wang Y, Katz JL. Identification of collagen encapsul-ation at the dentin/adhesive inter-face. J Adhes Dent. 2004. 6:91–95.4. Pashley DH, Ciucchi B, Sano H, Horner JA. Permeability of dentin to adhesive agents. Quintessence Int. 1993. 24:618–631.5. Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002. 62:447–456.
Article6. Armstrong SR, Vargas MA, Fang Q, Laffoon JE. Microtensile bond strength of a total-etch 3-step, total-etch 2-step, self-etch 2-step, and a self-etch 1-step dentin bonding system through 15-month water storage. J Adhes Dent. 2003. 5:47–56.7. Tay FR, Pashley DH, Suh BI, Carvalho RM, Itthagarun A. Single-step adhesives are permeable membranes. J Dent. 2002. 30:371–382.
Article8. Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003. 24:3795–3803.
Article9. Fukuda K, Nezu T, Terada Y. The effects of alcoholic com-pounds on the stability of type I collagen studied by differential scanning calorimetry. Dent Mater J. 2000. 19:221–228.
Article10. Perdigao J, Frankenberger R. Effect of solvent and rewetting time on dentin adhesion. Quintessence Int. 2001. 32:385–390.11. Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002. 81:472–476.
Article12. Tay FR, Pashley DH. Water treeing-a potential mechanism for degradation of dentin adhesives. Am J Dent. 2003. 16:6–12.13. Tay FR, Hashimoto M, Pashley DH, Peters MC, Lai SC, Yiu CK, Cheong C. Aging affects two modes of nanoleakage expression in bonded dentin. J Dent Res. 2003. 82:537–541.
Article14. Okuda M, Pereira PN, Nakajima M, Tagami J. Relationship between nanoleakage and long-term durability of dentin bonds. Oper Dent. 2001. 26:482–490.15. Sano H. Microtensile testing, nanoleakage, and biodegradation of resin-dentin bonds. J Dent Res. 2006. 85:11–14.
Article16. Sano H, Yoshiyama M, Ebisu S, Burrow MF, Takatsu T, Ciucchi B, Carvalho R, Pashley DH. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995. 20:160–167.17. Sano H, Yoshikawa T, Pereira PN, Kanemura N, Morigami M, Tagami J, Pashley DH. Long-term durability of dentin bonds made with a self-etching primer, in vivo. J Dent Res. 1999. 78:906–911.
Article18. Sano H, Shono T, Takatsu T, Hosoda H. Microporous dentin zone beneath resin-impregnated layer. Oper Dent. 1994. 19:59–64.19. Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995. 20:18–25.20. Maciel KT, Carvalho RM, Ringle RD, Preston CD, Russell CM, Pashley DH. The effects of acetone, ethanol, HEMA, and air on the stiffness of human decalcified dentin matrix. J Dent Res. 1996. 75:1851–1858.
Article21. Nakabayashi N, Watanabe A, Gendusa NJ. Dentin adhesion of "modified"4-META/MMA-TBB resin: function of HEMA. Dent Mater. 1992. 8:259–264.22. Pashley EL, Zhang Y, Lockwood PE, Rueggeberg FA, Pashley DH. Effects of HEMA on water evaporation from water-HEMA mixtures. Dent Mater. 1998. 14:6–10.
Article23. Bedran-de-Castro AK, Pereira PN, Pimenta LA, Thompson JY. Effect of thermal and mechanical load cycling on nanoleakage of Class II restorations. J Adhes Dent. 2004. 6:221–226.24. Bedran-de-Castro AK, Pereira PN, Pimenta LA, Thompson JY. Effect of thermal and mechanical load cycling on microtensile bond strength of a total-etch adhesive system. Oper Dent. 2004. 29:150–156.25. Anderson DJ. Measurement of stress in mastication. II. J Dent Res. 1956. 35:671–673.
Article26. Ausiello P, Davidson CL, Cascone P, DeGee AJ, Rengo S. Debonding of adhesively restored deep Class II MOD restorations after functional loading. Am J Dent. 1999. 12:84–88.27. Hakimeh S, Vaidyanathan J, Houpt ML, Vaidyanathan TK, Von Hagen S. Microleakage of compomer class V restorations: effect of load cycling, thermal cycling, and cavity shape differences. J Prosthet Dent. 2000. 83:194–203.
Article28. da cunha Mello FS, Feilzer AJ, de Gee AJ, Davidson CL. Sealing ability of eight resin bonding systems in a Class II restoration after mechanical fatiguing. Dent Mater. 1997. 13:372–376.
Article29. Prati C, Tao L, Simpson M, Pashley DH. Permeability and microleakage of Class II resin composite restorations. J Dent. 1994. 22:49–56.
Article30. Li H, Burrow MF, Tyas MJ. The effect of load cycling on the nanoleakage of dentin bonding systems. Dent Mater. 2002. 18:111–119.
Article31. Yap A, Stokes AN, Pearson GJ. An in vitro microleakage study of a new multi-purpose dental adhesive system. J Oral Rehabil. 1996. 23:302–308.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The nanoleakage patterns of different dentin adhesive systems
- Effect of intermediate resin hydrophilicity on bond strength of single step adhesive
- THE EFFECTS OF THERMAL CYCLING ON THE BOND STRENGTH OF SELF-CURING RESIN
- Effect of the additional application of a resin layer on dentin bonding using single-step adhesives
- Comparison of microleakage after load cycling for nanofilled composite resin fillings with or without flowable resin lining