Int J Stem Cells.
2014 Nov;7(2):79-86. 10.15283/ijsc.2014.7.2.79.
Possible Local Stem Cells Activation by Microcurrent Application in Experimentally Injured Soleus Muscle
- Affiliations
-
- 1Department of Histology, Faculty of Medicine, Cairo University, Cairo, Egypt. maha_kaah@yahoo.com
- KMID: 1974595
- DOI: http://doi.org/10.15283/ijsc.2014.7.2.79
Abstract
- BACKGROUND
Severe injuries in skeletal muscle result in muscle weakness that delays recovery and contribute to progressive decline in muscle function. Microcurrent therapy (MCT) is a novel treatment method used in soft tissue injury and tissue regeneration therapy. The regenerative capacity of skeletal muscle tissue resides in satellite cells, the quiescent adult stem cells. AIM: The present work aimed at investigating the relation between microcurrent therapy and local stem cells in regeneration of induced skeletal muscle injury in albino rat.
MATERIALS AND METHODS
Twenty six adult male albino rats were divided into Sham group, Injury group (I): subjected to soleus muscle injury and subdivided into subgroups I1 & I2 sacrificed 2 and 4 weeks after injury respectively. Microcurrent group (M): subjected to muscle injury and micro-current was applied. The animals were subdivided into subgroups M1 and M2 sacrificed 2 and 4 weeks after injury. Histological, immunohistochemical and morphometric studies were performed.
RESULTS
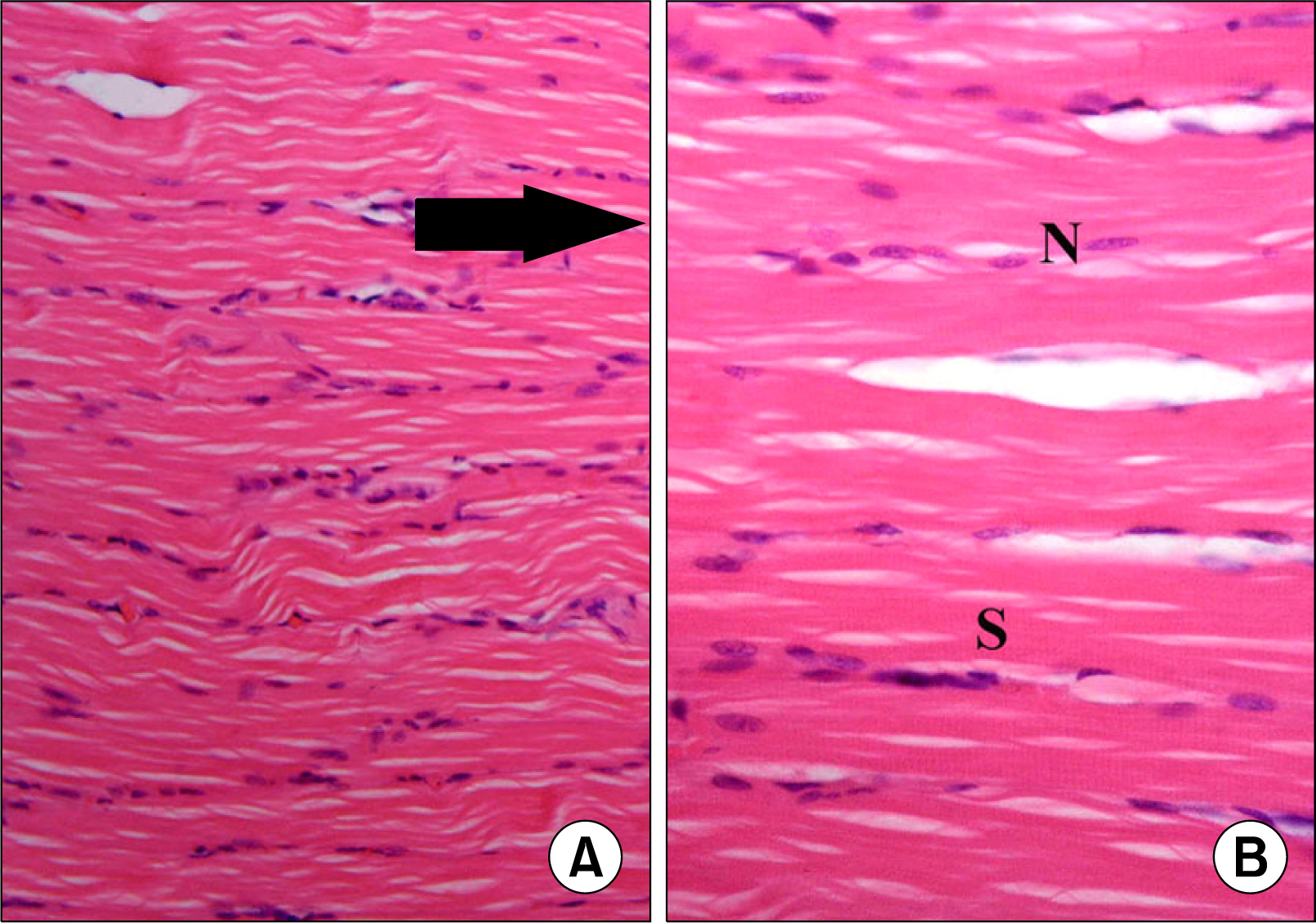
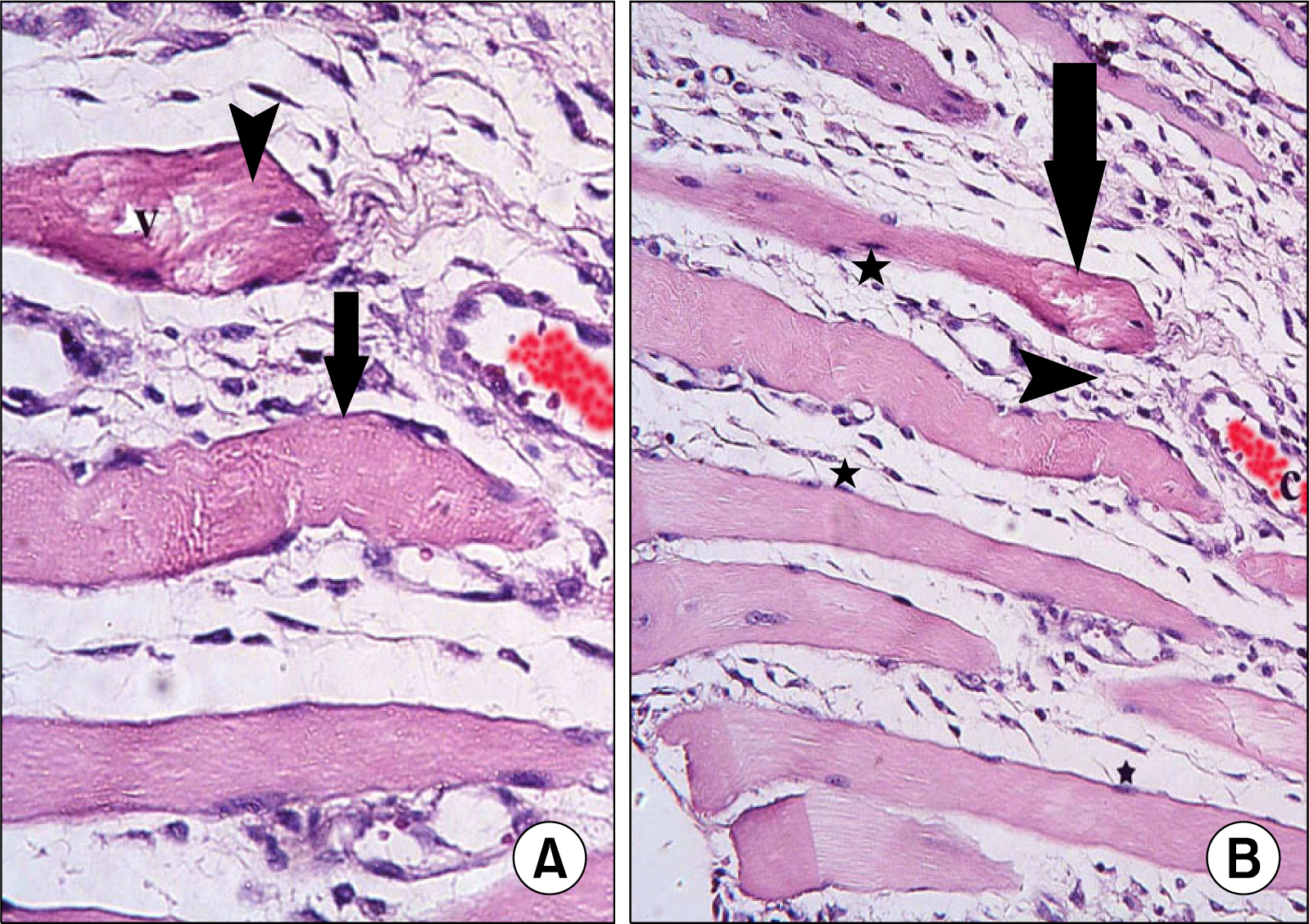
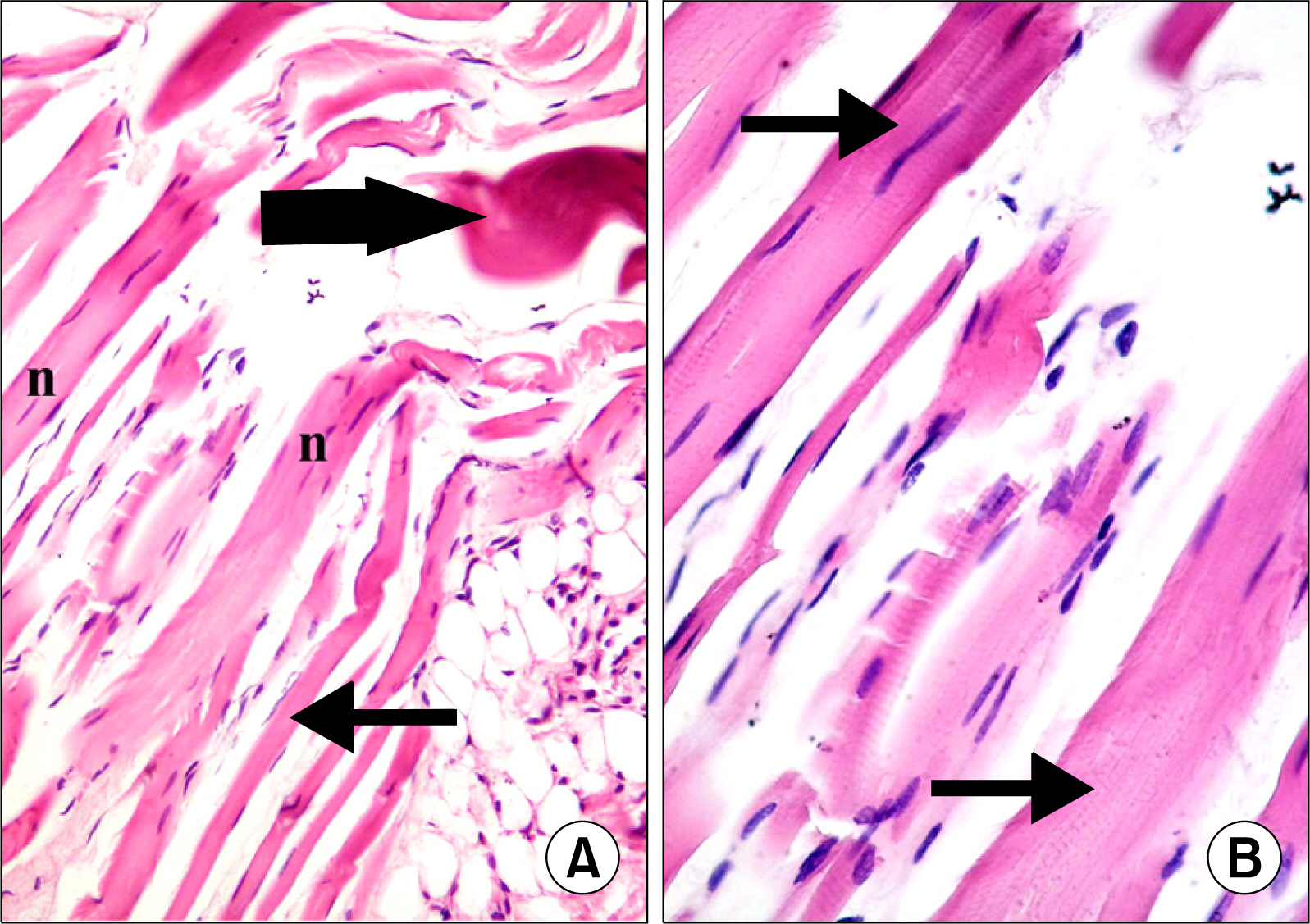
Atypical fibers widely separated by infiltrating cells and strong acidophilic sarcoplasm with focal vacuolations were found in injury group. In M1 subgroup few atypical fibers were found. In M2 subgroup multiple typical fibers were detected. A significant decrease in the mean area of atypical fibers, a significant increase in the mean area% of alpha SMA+ve cells and that of CD34+ve cells were found in microcurrent group compared to injury group.
CONCLUSIONS
A definite therapeutic effect of the microcurrent was found on induced skeletal muscle injury. This effect was proved to be related to satellite cell activation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Oliveira Fd, Bevilacqua LR, Anaruma CA, Boldrini Sde C, Liberti EA. Morphological changes in distant muscle fibers following thermal injury in Wistar rats. Acta Cir Bras. 2010. 25:525–528.
Article2. Gumerson JD, Michele DE. The dystrophin-glycoprotein complex in the prevention of muscle damage. J Biomed Biotechnol. 2011. 2011:210797.
Article3. Kim MY, Kwon DR, Lee HI. Therapeutic effect of micro-current therapy in infants with congenital muscular torticollis. PM R. 2009. 1:736–739.
Article4. Lee BY, Al-Waili N, Stubbs D, Wendell K, Butler G, Al-Waili T, Al-Waili A. Ultra-low microcurrent in the management of diabetes mellitus, hypertension and chronic wounds: report of twelve cases and discussion of mechanism of action. Int J Med Sci. 2009. 7:29–35.
Article5. Aliyev RM, Geiger G. Cell-stimulation therapy of lateral epicondylitis with frequency-modulated low-intensity electric current. Bull Exp Biol Med. 2012. 152:653–655.
Article6. Ieronimakis N, Balasundaram G, Rainey S, Srirangam K, Yablonka-Reuveni Z, Reyes M. Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PLoS One. 2010. 5:e10920–e10935.
Article7. Armand AS, Laziz I, Djeghloul D, Lécolle S, Bertrand AT, Biondi O, De Windt LJ, Chanoine C. Apoptosis-inducing factor regulates skeletal muscle progenitor cell number and muscle phenotype. PLoS One. 2011. 6:e27283–e27307.
Article8. Riuzzi F, Sorci G, Beccafico S, Donato R. S100B engages RAGE or bFGF/FGFR1 in myoblasts depending on its own concentration and myoblast density. Implications for muscle regeneration. PLoS One. 2012. 7:e28700–e28717.
Article9. Lee YS, Kwon ST, Kim JO, Choi ES. Serial MR imaging of intramuscular hematoma: experimental study in a rat model with the pathologic correlation. Korean J Radiol. 2011. 12:66–77.
Article10. Passarini Junior JR, Gaspi FO, Neves LM, Esquisatto MA, Santos GM, Mendonça FA. Application of Jatropha curcas L. seed oil (Euphorbiaceae) and microcurrent on the healing of experimental wounds in Wistar rats. Acta Cir Bras. 2012. 27:441–447.
Article11. Mehmandoust FG, Torkaman G, Firoozabadi M, Talebi G. Anodal and cathodal pulsed electrical stimulation on skin wound healing in guinea pigs. J Rehabil Res Dev. 2007. 44:611–618.
Article12. Curtis D, Fallows S, Morris M, McMakin C. The efficacy of frequency specific microcurrent therapy on delayed onset muscle soreness. J Bodyw Mov Ther. 2010. 14:272–279.
Article13. Kiernan JA. Histological and histochemical methods: theory and Practice. 3rd ed. London: Hodder Arnold Publishers;2001. 111–162.14. Elia A, Charalambous F, Georgiades P. New phenotypic aspects of the decidual spiral artery wall during early post-implantation mouse pregnancy. Biochem Biophys Res Commun. 2011. 416:211–216.
Article15. Zhou JH, Cao LH, Liu JB, Zheng W, Liu M, Luo RZ, Han F, Li AH. Quantitative assessment of tumor blood flow in mice after treatment with different doses of an antiangiogenic agent with contrast-enhanced destruction-replenishment US. Radiology. 2011. 259:406–413.
Article16. Pasut A, Oleynik P, Rudnicki MA. Isolation of muscle stem cells by fluorescence activated cell sorting cytometry. Methods Mol Biol. 2012. 798:53–64.
Article17. Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010. 19:237–270.
Article18. Crawford RS, Albadawi H, Atkins MD, Jones JJ, Conrad MF, Austen WG Jr, Fink MP, Watkins MT. Postischemic treatment with ethyl pyruvate prevents adenosine triphosphate depletion, ameliorates inflammation, and decreases thrombosis in a murine model of hind-limb ischemia and reperfusion. J Trauma. 2011. 70:103–110.
Article19. Mu X, Peng H, Pan H, Huard J, Li Y. Study of muscle cell dedifferentiation after skeletal muscle injury of mice with a Cre-Lox system. PLoS One. 2011. 6:e16699–e16707.
Article20. Lazarenko NN, Gerasimenko MIu. The application of multichannel electrostimulation and nivalin electrophoresis for the rehabilitative treatment of the patient following plastic surgery in the facial region. Vopr Kurortol Fizioter Lech Fiz Kult. 2011. (5):39–44.21. Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012. 148:112–125.
Article22. Robson LG, Di Foggia V, Radunovic A, Bird K, Zhang X, Marino S. Bmi1 is expressed in postnatal myogenic satellite cells, controls their maintenance and plays an essential role in repeated muscle regeneration. PLoS One. 2011. 6:e27116–e27126.
Article23. François S, D'Orlando C, Fatone T, Touvier T, Pessina P, Meneveri R, Brunelli S. Necdin enhances myoblasts survival by facilitating the degradation of the mediator of apoptosis CCAR1/CARP1. PLoS One. 2012. 7:e43335–e43345.
Article24. Mu X, Urso ML, Murray K, Fu F, Li Y. Relaxin regulates MMP expression and promotes satellite cell mobilization during muscle healing in both young and aged mice. Am J Pathol. 2010. 177:2399–2410.
Article25. Di Foggia V, Robson L. Isolation of satellite cells from single muscle fibers from young, aged, or dystrophic muscles. Methods Mol Biol. 2012. 916:3–14.
Article26. Alfaro LA, Dick SA, Siegel AL, Anonuevo AS, McNagny KM, Megeney LA, Cornelison DD, Rossi FM. CD34 promotes satellite cell motility and entry into proliferation to facilitate efficient skeletal muscle regeneration. Stem Cells. 2011. 29:2030–2041.
Article27. Zhao X, Huang L. Cardiac stem cells: A promising treatment option for heart failure. Exp Ther Med. 2013. 5:379–383.
Article28. Rajabi-Zeleti S, Jalili-Firoozinezhad S, Azarnia M, Khayyatan F, Vahdat S, Nikeghbalian S, Khademhosseini A, Baharvand H, Aghdami N. The behavior of cardiac progenitor cells on macroporous pericardium-derived scaffolds. Biomaterials. 2014. 35:970–982.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect Exercise on the Mass and Relative Muscle Weigth of Atrophied Soleus Muscles of Rats
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- Effect of exercise training following hypokinesia on the length and circumference of atrophied soleus and plantaris muscle in rats
- The Co-existence of the Gastrocnemius Tertius and Accessory Soleus Muscles
- Comparison of Regeneration Effects of Direct and Alternating Microcurrent Therapy on Atrophied Calf Muscle in a Rabbit