Clin Exp Otorhinolaryngol.
2015 Mar;8(1):13-19. 10.3342/ceo.2015.8.1.13.
Donor-Site Morbidity Following Minimally Invasive Costal Cartilage Harvest Technique
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Chonnam National University Medical School, Gwangju, Korea. victocho@hanmail.net, choyb@chonnam.ac.kr
- KMID: 1973482
- DOI: http://doi.org/10.3342/ceo.2015.8.1.13
Abstract
OBJECTIVES
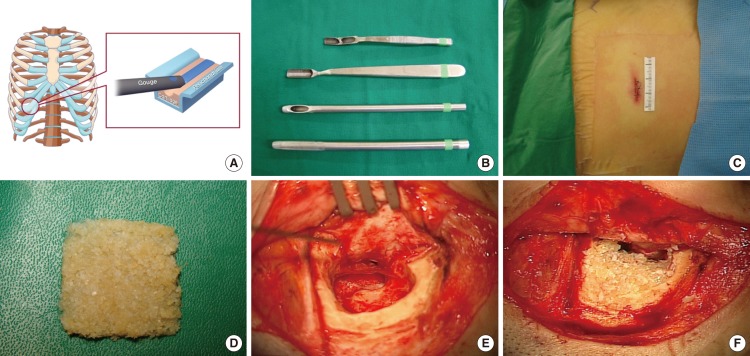
Autologous costal cartilage is a promising alternative for mastoid obliteration. However, donor-site morbidities of the chest wall limit the use of this graft. To address this issue, we have developed a minimally-invasive technique of harvesting costal cartilage and report donor site morbidity associated with the procedure.
METHODS
Donor site morbidities were evaluated for 151 patients who underwent costal cartilage harvest, canal wall down mastoidectomy, and mastoid obliteration. Pain and cosmetic concern were evaluated via visual analogue scale (VAS). Scars were evaluated via the modified Vancouver Scar Scale (VSS) and the Patient and Observer Scar Assessment Scale (POSAS). Postoperative complications were assessed during the follow-up period.
RESULTS
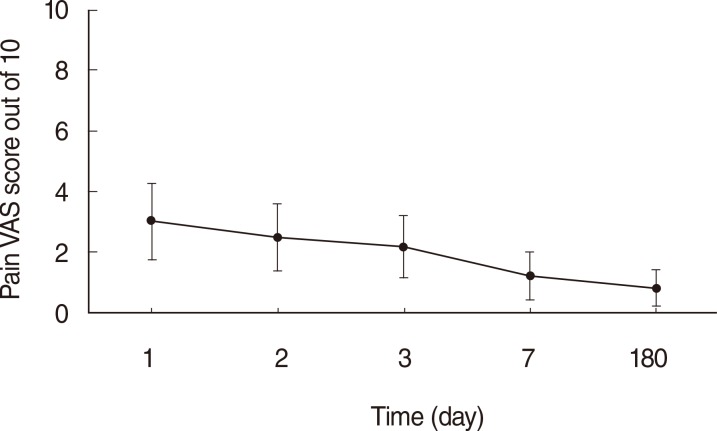
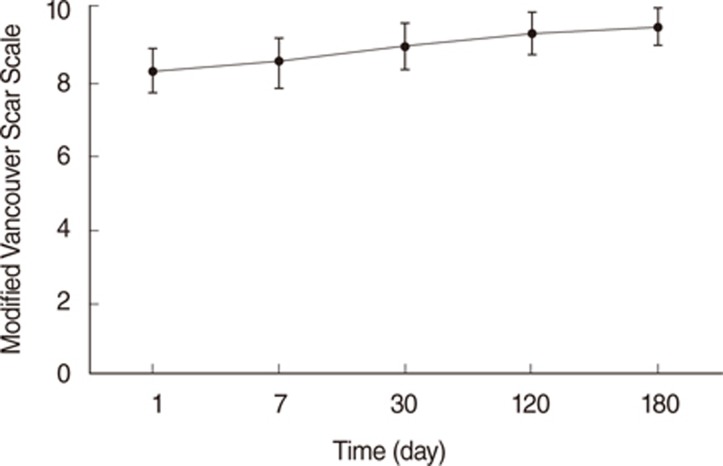
The mean duration of noticeable pain was 5.3 days post operation. The mean VAS score for pain was 3.0 of 10 on the first day after the operation and gradually declined. At the 6 months post operation, the mean VAS cosmetic score at the costal cartilage harvest site was 0.6 of 10. The mean VSS score was 9.5 out of 10 total, and the mean POSAS score was 23.27 out of 110 total.
CONCLUSION
The minimally-invasive chopped costal cartilage harvest technique resulted in acceptable pain, cosmetic concern, and postoperative complications for most patients. There were no major postoperative complications. Costal cartilage is an acceptable donor for mastoid obliteration in canal wall down mastoidectomy, especially in the context of the extremely low donor site morbidity of the minimally-invasive technique presented in the study.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Hypoxia Differentially Affects Chondrogenic Differentiation of Progenitor Cells from Different Origins
Mira Hammad, Alexis Veyssiere, Sylvain Leclercq, Vincent Patron, Catherine Baugé, Karim Boumédiene
Int J Stem Cells. 2023;16(3):304-314. doi: 10.15283/ijsc21242.
Reference
-
1. Males AG, Gray RF. Mastoid misery: quantifying the distress in a radical cavity. Clin Otolaryngol Allied Sci. 1991; 2. 16(1):12–14. PMID: 2032350.
Article2. Whittemore KR Jr, Merchant SN, Rosowski JJ. Acoustic mechanisms: canal wall-up versus canal wall-down mastoidectomy. Otolaryngol Head Neck Surg. 1998; 6. 118(6):751–761. PMID: 9627232.3. Shelton C, Sheehy JL. Tympanoplasty: review of 400 staged cases. Laryngoscope. 1990; 7. 100(7):679–681. PMID: 2362526.4. Takahashi H, Iwanaga T, Kaieda S, Fukuda T, Kumagami H, Takasaki K, et al. Mastoid obliteration combined with soft-wall reconstruction of posterior ear canal. Eur Arch Otorhinolaryngol. 2007; 8. 264(8):867–871. PMID: 17340129.
Article5. Gantz BJ, Wilkinson EP, Hansen MR. Canal wall reconstruction tympanomastoidectomy with mastoid obliteration. Laryngoscope. 2005; 10. 115(10):1734–1740. PMID: 16222186.
Article6. Kim SH, Kang MK, Ahn JK, Han CS, Gu TW. Epitympanoplasty with mastoid obliteration technique in middle ear surgery:12-year result. J Int Adv Otol. 2009; 5(1):24–30.7. Shea MC Jr, Gardner G Jr, Simpson ME. Mastoid obliteration using homogenous bone chips and autogenous bone paste. Trans Am Acad Ophthalmol Otolaryngol. 1972; Jan-Feb. 76(1):160–172. PMID: 4337026.8. Grote JJ. Results of cavity reconstruction with hydroxyapatite implants after 15 years. Am J Otol. 1998; 9. 19(5):565–568. PMID: 9752961.9. Lee HB, Lim HJ, Cho M, Yang SM, Park K, Park HY, et al. Clinical significance of β-tricalcium phosphate and polyphosphate for mastoid cavity obliteration during middle ear surgery: human and animal study. Clin Exp Otorhinolaryngol. 2013; 9. 6(3):127–134. PMID: 24069514.
Article10. Jang CH, Park H, Cho YB, Song CH. Mastoid obliteration using a hyaluronic acid gel to deliver a mesenchymal stem cells-loaded demineralized bone matrix: an experimental study. Int J Pediatr Otorhinolaryngol. 2008; 11. 72(11):1627–1632. PMID: 18786734.
Article11. Leatherman BD, Dornhoffer JL. The use of demineralized bone matrix for mastoid cavity obliteration. Otol Neurotol. 2004; 1. 25(1):22–25. PMID: 14724487.
Article12. Cho SW, Cho YB, Cho HH. Mastoid obliteration with silicone blocks after canal wall down mastoidectomy. Clin Exp Otorhinolaryngol. 2012; 3. 5(1):23–27. PMID: 22468198.
Article13. Black B. Mastoidectomy elimination. Laryngoscope. 1995; 12. 105(12 Pt 2 Suppl 76):1–30. PMID: 7500804.
Article14. Black B. Mastoidectomy elimination: obliterate, reconstruct, or ablate? Am J Otol. 1998; 9. 19(5):551–557. PMID: 9752959.15. Leatherman BD, Dornhoffer JL, Fan CY, Mukunyadzi P. Demineralized bone matrix as an alternative for mastoid obliteration and posterior canal wall reconstruction: results in an animal model. Otol Neurotol. 2001; 11. 22(6):731–736. PMID: 11698788.
Article16. Moon BJ, Lee HJ, Jang YJ. Outcomes following rhinoplasty using autologous costal cartilage. Arch Facial Plast Surg. 2012; May-Jun. 14(3):175–180. PMID: 22801761.
Article17. Siegert R. Combined reconstruction of congenital auricular atresia and severe microtia. Laryngoscope. 2003; 11. 113(11):2021–2027. PMID: 14603067.
Article18. Cotton RT, Myer CM 3rd, O'Connor DM, Smith ME. Pediatric laryngotracheal reconstruction with cartilage grafts and endotracheal tube stenting: the single-stage approach. Laryngoscope. 1995; 8. 105(8 Pt 1):818–821. PMID: 7630293.
Article19. Uppal RS, Sabbagh W, Chana J, Gault DT. Donor-site morbidity after autologous costal cartilage harvest in ear reconstruction and approaches to reducing donor-site contour deformity. Plast Reconstr Surg. 2008; 6. 121(6):1949–1955. PMID: 18520880.
Article20. Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995; Sep-Oct. 16(5):535–538. PMID: 8537427.
Article21. Draaijers LJ, Tempelman FR, Botman YA, Tuinebreijer WE, Middelkoop E, Kreis RW, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004; 6. 113(7):1960–1965. PMID: 15253184.
Article22. Rasp G, Staudenmaier R, Ledderose H, Kastenbauer E. Autologous rib cartilage harvesting: operative procedure and postoperative pain reduction. Laryngorhinootologie. 2000; 3. 79(3):155–159. PMID: 10763173.23. Chepla KJ, Salgado CJ, Tang CJ, Mardini S, Evans KK. Late complications of chest wall reconstruction: management of painful sternal nonunion. Semin Plast Surg. 2011; 2. 25(1):98–106. PMID: 22294948.
Article24. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976; 6. 2(2):175–184. PMID: 1026900.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Usefulness of Allogenous Costal Cartilage Graft for Correction of Short Nose and Tip Plasty
- Growth Effect of the Chest Wall after Costal Cartilage Harvesting for Correction of Congenital Microtia
- Using Rib Bone Turnover Technique, Prevention of Chest Wall Depression after Microtia Reconstruction
- Lyophilized allogeneic costal cartilage graft for septorhinoplasty
- 10th Rib Cartilage: Another Option of the Costal Cartilage Graft for Rhinoplasty