Cancer Res Treat.
2012 Sep;44(3):157-165.
Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study
- Affiliations
-
- 1Department of Pathology, Wonju Christian Hospital, Wonju, Korea.
- 2Department of Pathology, Inha University Hospital, Incheon, Korea.
- 3Department of Pathology, Kangbuk Samsung Medical Center, Seoul, Korea. jhpath.sohn@samsung.com
- 4Department of Pathology, Asan Medical Center, Seoul, Korea.
- 5Department of Pathology, Samsung Medical Center, Seoul, Korea.
- 6Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- 7Department of Pathology, Severance Hospital, Seoul, Korea.
- 8Department of Pathology, National Cancer Center, Goyang, Korea.
- 9Department of Pathology, Pusan National University Hospital, Busan, Korea.
- 10Department of Pathology, Chonnam National University Hospital, Gwangju, Korea.
- 11Department of Pathology, Kosin University Gospel Hospital, Busan, Korea.
- 12Department of Pathology, Seoul St. Mary's Hospital, Seoul, Korea.
- 13Department of Pathology, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- 14Department of Pathology, Soonchunhyang University Hospital, Seoul, Korea.
- 15Department of Pathology, Yeungnam University Hospital, Daegu, Korea.
- 16Department of Pathology, Daegu Fatima Hospital, Daegu, Korea.
- 17Department of Pathology, Dong-A University Hospital, Busan, Korea.
- 18Department of Pathology, Busan Paik Hospital, Busan, Korea.
- 19Department of Pathology, Daegu Catholic University Medical Center, Daegu, Korea.
- 20Department of Pathology, Konyang University Hospital, Daejeon, Korea.
- 21Department of Pathology, Seoul Paik Hospital, Seoul, Korea.
- 22Department of Pathology, Kyunghee University Hospital, Seoul, Korea.
- 23Department of Pathology, Gangnam Severerance Hospital, Seoul, Korea.
- 24Department of Pathology, Kyungpook National University Hospital, Daegu, Korea.
- 25Department of Pathology, Ilsan Paik Hospital, Goyang, Korea.
- 26Department of Pathology, Chonbuk National University Hospital, Jeonju, Korea.
- 27Department of Pathology, Chungnam National University Hospital, Daejeon, Korea.
- 28Department of Preventive Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
Abstract
- PURPOSE
As a result of various independently proposed nomenclatures and classifications, there is confusion in the diagnosis and prediction of biological behavior of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). A comprehensive nationwide study is needed in order to understand the biological characteristics of GEP-NETs in Korea.
MATERIALS AND METHODS
We collected 4,951 pathology reports from 29 hospitals in Korea between 2000 and 2009. Kaplan-Meier survival analysis was used to determine the prognostic significance of clinicopathological parameters.
RESULTS
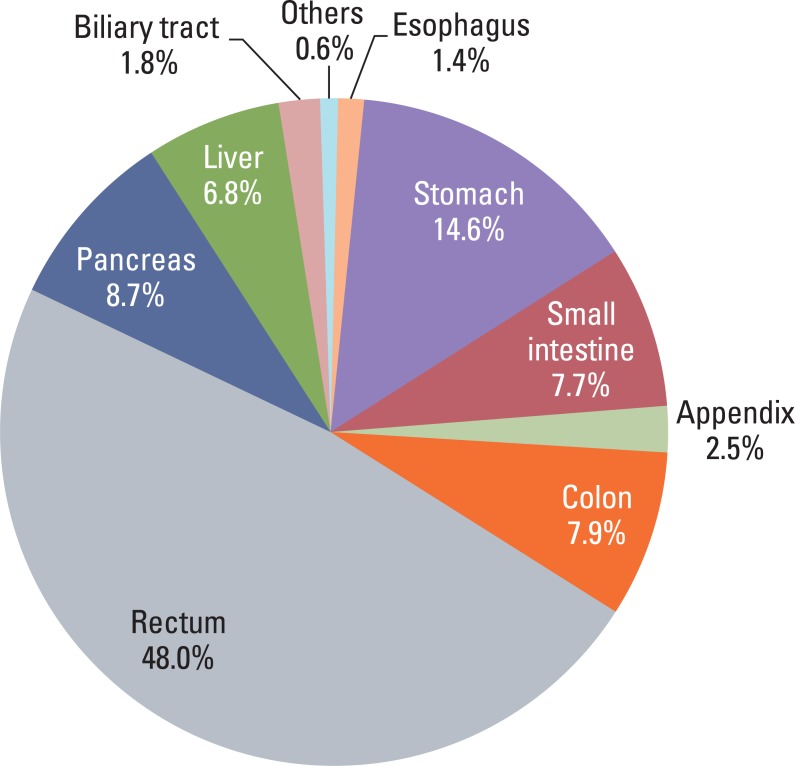
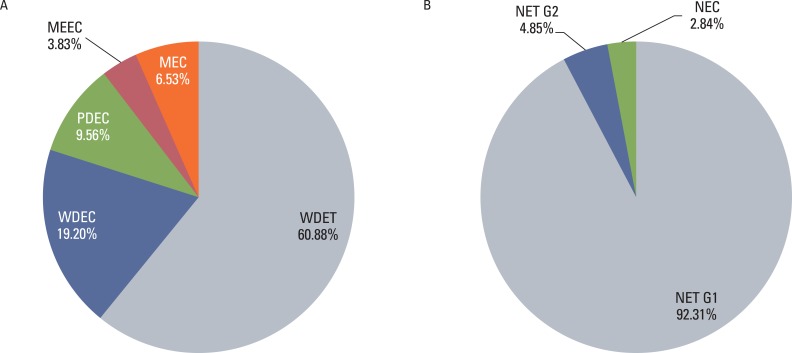
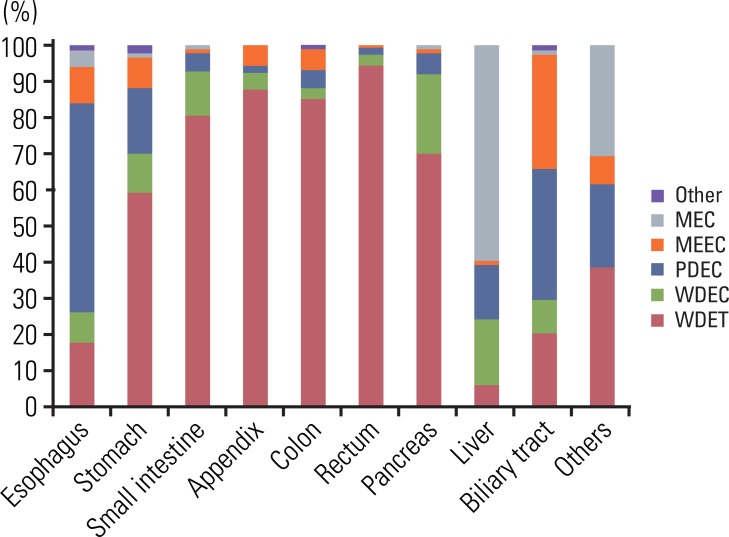
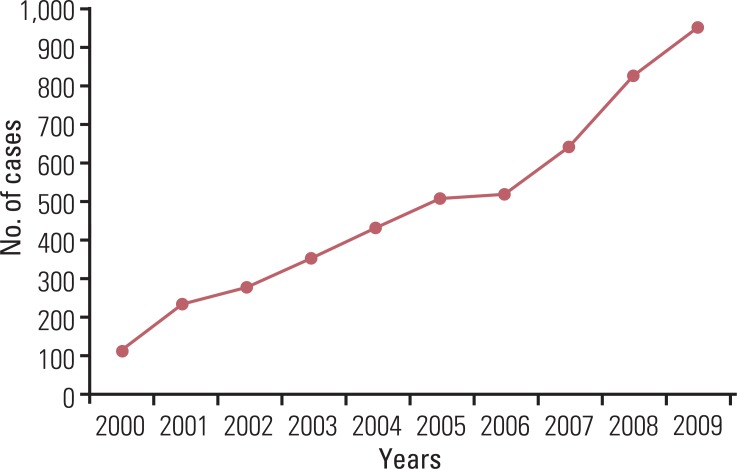
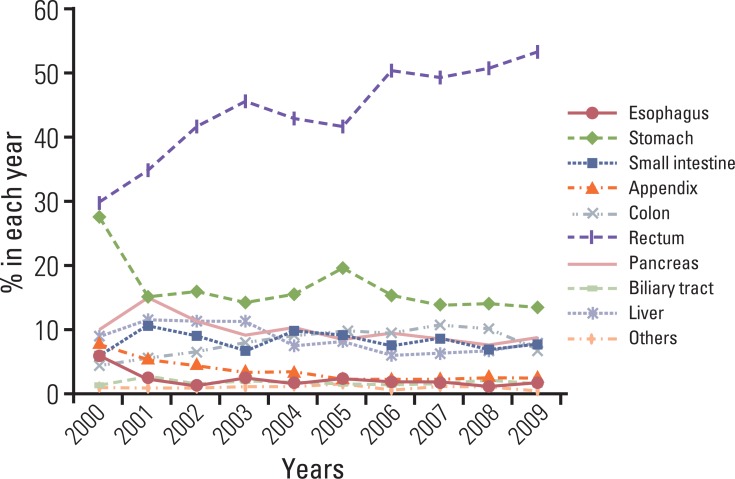
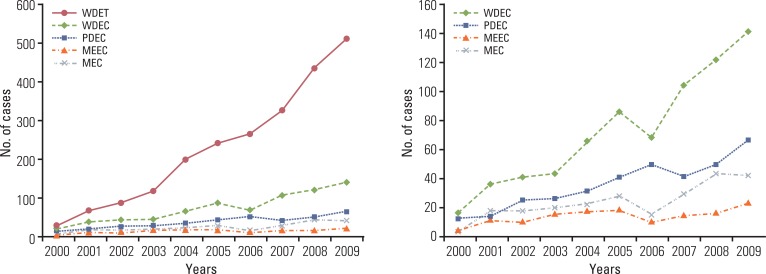
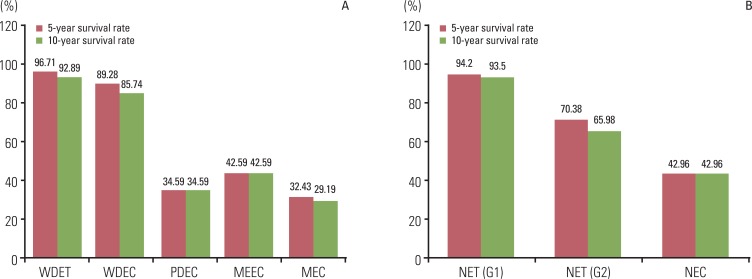
Although the GEP-NET is a relatively rare tumor in Korea, its incidence has increased during the last decade, with the most significant increase found in the rectum. The 10-year survival rate for well-differentiated endocrine tumor was 92.89%, in contrast to 85.74% in well differentiated neuroendocrine carcinoma and 34.59% in poorly differentiated neuroendocrine carcinoma. Disease related death was most common in the biliary tract (62.2%) and very rare in the rectum (5.2%). In Kaplan-Meier survival analysis, tumor location, histological classification, extent, size, mitosis, Ki-67 labeling index, synaptophysin expression, lymphovascular invasion, perineural invasion, and lymph node metastasis showed prognostic significance (p<0.05), however, chromogranin expression did not (p=0.148). The 2000 and 2010 World Health Organization (WHO) classification proposals were useful for prediction of the prognosis of GEP-NET.
CONCLUSION
The incidence of GEP-NET in Korea has shown a remarkable increase during the last decade, however, the distribution of tumors in the digestive system differs from that of western reports. Assessment of pathological parameters, including immunostaining, is crucial in understanding biological behavior of the tumor as well as predicting prognosis of patients with GEP-NET.
MeSH Terms
-
Biliary Tract
Carcinoma, Neuroendocrine
Digestive System
Humans
Incidence
Intestinal Neoplasms
Korea
Lymph Nodes
Mitosis
Neoplasm Metastasis
Neuroendocrine Tumors
Pancreatic Neoplasms
Population Characteristics
Prognosis
Rectum
Stomach Neoplasms
Survival Rate
Synaptophysin
World Health Organization
Intestinal Neoplasms
Neuroendocrine Tumors
Pancreatic Neoplasms
Stomach Neoplasms
Synaptophysin
Figure
Reference
-
1. Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004; 1014:13–27. PMID: 15153416.2. Kloppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009; 90:162–166. PMID: 19060454.
Article3. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010; 39:707–712. PMID: 20664470.4. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 2010. 4th ed. Lyon: International Agency for Research on Cancer.5. Kloppel G, Rindi G, Anlauf M, Perren A, Komminoth P. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007; 451(Suppl 1):S9–S27. PMID: 17684761.
Article6. Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen RT, Goldsmith SJ, et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the jejunum, ileum, appendix, and cecum. Pancreas. 2010; 39:753–766. PMID: 20664473.7. Washington MK, Tang LH, Berlin J, Branton PA, Burgart LJ, Carter DK, et al. Protocol for the examination of specimens from patients with neuroendocrine tumors (carcinoid tumors) of the colon and rectum. Arch Pathol Lab Med. 2010; 134:176–180. PMID: 20121603.
Article8. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.
Article9. Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gonen M, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010; 34:300–313. PMID: 20118772.
Article10. Vinik AI, Woltering EA, Warner RR, Caplin M, O'Dorisio TM, Wiseman GA, et al. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010; 39:713–734. PMID: 20664471.
Article11. Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006; 449:395–401. PMID: 16967267.
Article12. Fritz A, Percy C, Jack A, Shanmugaratnam S, Sobin L, Parkin DM, et al. International classification of diseases for oncology (ICD-O). 2000. 3rd ed. Geneva: World Health Organization.13. Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004; 240:117–122. PMID: 15213627.
Article14. Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Diaz-Perez JA, Martinez Del Prado MP, Alonso Orduna V, et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol. 2010; 21:1794–1803. PMID: 20139156.
Article15. Rothenstein J, Cleary SP, Pond GR, Dale D, Gallinger S, Moore MJ, et al. Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am J Clin Oncol. 2008; 31:64–70. PMID: 18376230.16. Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010; 45:234–243. PMID: 20058030.
Article17. Lee H, Choi J, An JS, Kim H, Shin BK, Kim A, et al. The clinicopathological characteristics of gastrointestinal neuroendocrine tumors: an analysis of 65 cases. Korean J Pathol. 2007; 41:149–157.18. Chetty R. An overview of practical issues in the diagnosis of gastroenteropancreatic neuroendocrine pathology. Arch Pathol Lab Med. 2008; 132:1285–1289. PMID: 18684027.
Article19. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 2010. 7th ed. New York: Springer.20. Arnold R. Endocrine tumours of the gastrointestinal tract. Introduction: definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract Res Clin Gastroenterol. 2005; 19:491–505. PMID: 16183523.21. Williams ED, Siebenmann RE, Sobin LH. Histological typing of endocrine tumours. 1980. Geneva: World Health Organization.22. Colonoscopy Study Group of Korean Society of Coloproctology. Clinical characteristics of colorectal carcinoid tumors. J Korean Soc Coloproctol. 2011; 27:17–20. PMID: 21431092.23. Jernman J, Valimaki MJ, Louhimo J, Haglund C, Arola J. The novel WHO 2010 classification for gastrointestinal neuroendocrine tumours correlates well with the metastatic potential of rectal neuroendocrine tumours. Neuroendocrinology. 2012; 95:317–324. PMID: 22327359.
Article24. Hirata I, Akutagawa H, Nishikawa T, Yasumoto S, Sanomura M, Egashira Y, et al. Clinicopathological study of colorectal carcinoid tumor, focusing on the indication for endoscopic mucosal resection (EMR). Stormach Intest. 2005; 40:182–193.25. Tabata H, Matsumoto T, Kuwano Y, Yao T, Iida M. Rectal carcinoid tumor 10 mm in diameter accompanied by multiple liver metastases, report of a case. Stormach Intest. 2005; 40:215–220.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Ultrasound in Gastroenteropancreatic Neuroendocrine Tumors
- Gastroenteropancreatic Neuroendocrine Tumor with Hepatic Metastasis Misdiagnosed as Hepatocellular Carcinoma
- Clinical Significance of Protein Expression of Cyclooxygenase-2 and Somatostatin Receptors in Gastroenteropancreatic Neuroendocrine Tumors
- p27 Loss Is Associated with Poor Prognosis in Gastroenteropancreatic Neuroendocrine Tumors
- Systemic Treatment of Advanced Gastroenteropancreatic Neuroendocrine Tumors in Korea: Literature Review and Expert Opinion