Prog Med Phys.
2014 Sep;25(3):128-138. 10.14316/pmp.2014.25.3.128.
Verification of Gated Radiation Therapy: Dosimetric Impact of Residual Motion
- Affiliations
-
- 1Loma Linda University Medical Center, Loma Linda, CA, USA. medicphys@hotmail.com
- 2East Carolina University, NC, USA.
- KMID: 1910544
- DOI: http://doi.org/10.14316/pmp.2014.25.3.128
Abstract
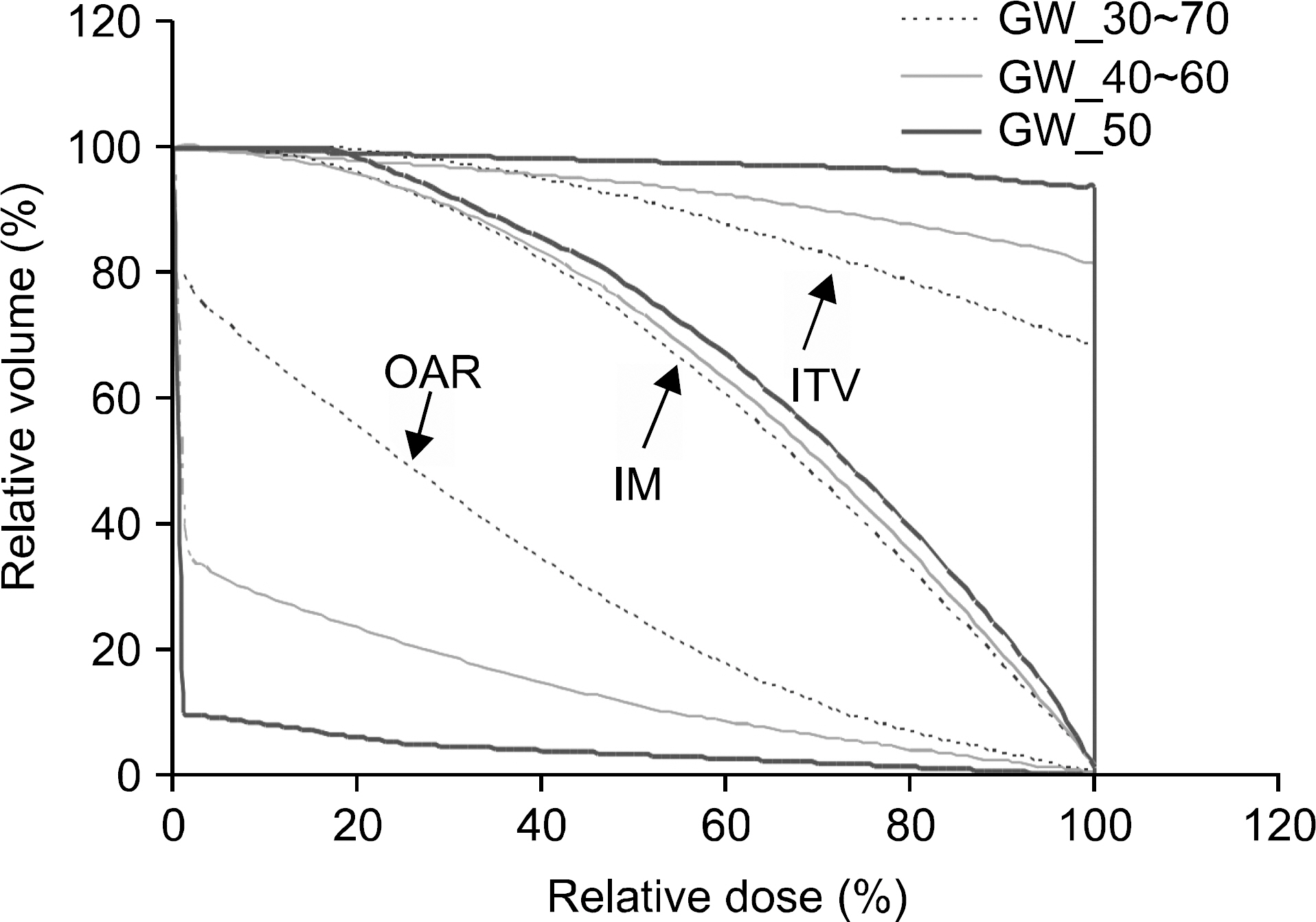
- In gated radiation therapy (gRT), due to residual motion, beam delivery is intended to irradiate not only the true extent of disease, but also neighboring normal tissues. It is desired that the delivery covers the true extent (i.e. clinical target volume or CTV) as a minimum, although target moves under dose delivery. The objectives of our study are to validate if the intended dose is surely delivered to the true target in gRT and to quantitatively understand the trend of dose delivery on it and neighboring normal tissues when gating window (GW), motion amplitude (MA), and CTV size changes. To fulfill the objectives, experimental and computational studies have been designed and performed. A custom-made phantom with rectangle- and pyramid-shaped targets (CTVs) on a moving platform was scanned for four-dimensional imaging. Various GWs were selected and image integration was performed to generate targets (internal target volume or ITV) for planning that included the CTVs and internal margins (IM). The planning was done conventionally for the rectangle target and IMRT optimization was done for the pyramid target. Dose evaluation was then performed on a diode array aligned perpendicularly to the gated beams through measurements and computational modeling of dose delivery under motion. This study has quantitatively demonstrated and analytically interpreted the impact of residual motion including penumbral broadening for both targets, perturbed but secured dose coverage on the CTV, and significant doses delivered in the neighboring normal tissues. Dose volume histogram analyses also demonstrated and interpreted the trend of dose coverage: for ITV, it increased as GW or MA decreased or CTV size increased; for IM, it increased as GW or MA decreased; for the neighboring normal tissue, opposite trend to that of IM was observed. This study has provided a clear understanding on the impact of the residual motion and proved that if breathing is reproducible gRT is secure despite discontinuous delivery and target motion. The procedures and computational model can be used for commissioning, routine quality assurance, and patient-specific validation of gRT. More work needs to be done for patient-specific dose reconstruction on CT images.
MeSH Terms
Figure
Reference
-
References
1. Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 3874–900 (. 2006.2. Berbeco RI, Nishioka S, Shirato H, Chen GTY, Jiang SB. Residual motion of lung tumours in gated radiotherapy with external respiratory surrogates. Phys Med Biol. 50(16):3655–67. 2005.
Article3. Hugo GD, Agazaryan N, Solberg TD. An evaluation of gating window size, delivery method, and composite field dosimetry of respiratory-gated IMRT. Med Phys. 29(11):2517. 2002.
Article23. ICRU-62. Prescribing, recording and reporting photon beam therapy (Supplement to ICRU Report 50). 1999.5. Kubo HD, Wang L. Compatibility of Varian 2100C gated operations with enhanced dynamic wedge and IMRT dose delivery. Med Phys. 27(8):1732. 2000.
Article6. Hugo GD, Agazaryan N, Solberg TD. The effects of tumor motion on planning and delivery of respiratory-gated IMRT. Med Phys. 30(6):1052. 2003.
Article7. Li XA, Stepaniak C, Gore E. Technical and dosimetric aspects of respiratory gating using a pressure-sensor motion monitoring system. Med Phys. 33(1):145. 2006.
Article8. Dietrich L, Tücking T, Nill S, Oelfke U. Compensation for respiratory motion by gated radiotherapy: an experimental study. Phys Med Biol. 50(10):2405–14. 2005.
Article9. Nelms BE, Ehler E, Bragg H, Tomé W a: Quality assurance device for four-dimensional IMRT or SBRT and respiratory gating using patient-specific intrafraction motion kernels. J Appl Clin Med Phys. 8(4):2683. 2007.10. Duan J, Shen S, Fiveash JB, et al. Dosimetric effect of respiration-gated beam on IMRT delivery. Med Phys. 30(8):2241. 2003.
Article11. Létourneau D, Gulam M, Yan D, Oldham M, Wong JW. Evaluation of a 2D diode array for IMRT quality assurance. Radiother Oncol. 70(2):199–206. 2004.
Article12. George R, Chung TD, Vedam SS, et al. Audiovisual biofeedback for respiratory-gated radiotherapy: impact of audio instruction and audio-visual biofeedback on respiratory-gated radiotherapy. Int J Radiat Oncol Biol Phys. 65(3):924–33. 2006.
Article13. Nelms BE, Simon JA. A survey on planar IMRT QA analysis. J Appl Clin Med Phys. 8(3):2448. 2007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitative Evaluation of Gated Radiation Therapy Using Gamma Index Analysis
- Dosimetric Analysis of Respiratory-Gated RapidArc with Varying Gating Window Times
- Development of Movement Analysis Program and Its Feasibility Test in Streotactic Body Radiation Threrapy
- Quasi-breath-hold (QBH) Biofeedback in Gated 3D Thoracic MRI: Feasibility Study
- Respiratory Motion Pattern Analysis of Lung Cancer Patient with Gated Treatment: A Preliminary Study