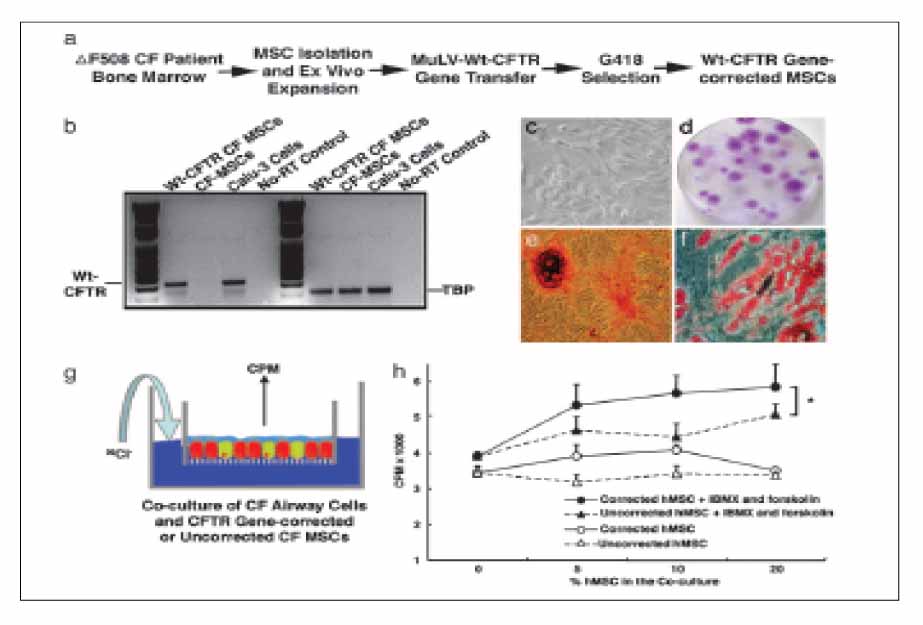
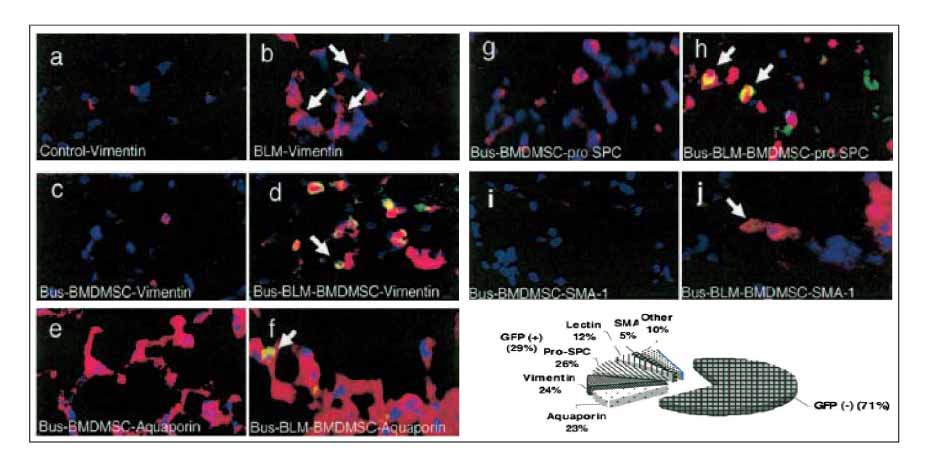
Tuberc Respir Dis.
2007 Aug;63(2):121-127. 10.4046/trd.2007.63.2.121.
Stem Cells in Respiratory Diseases
- Affiliations
-
- 1Department of Internal Medicine, Soonchunhyang University Hospital, Bucheon, Korea. jas877@schbc.ac.kr
- KMID: 1910090
- DOI: http://doi.org/10.4046/trd.2007.63.2.121
Abstract
- No abstract available.
MeSH Terms
Figure
Reference
-
1. Sanders RC Jr, Slayton WB, Cogle CR, Fisher RC, Scott EW. Stem cell research. Paediatr Respir Rev. 2006. 7:135–140.2. Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005. 172:854–860.3. Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, et al. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells. 2006. 24:1806–1813.4. Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, et al. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol. 2005. 32:87–92.5. Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005. 102:186–191.6. Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005. 33:145–152.7. Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006. 3:718–725.8. Al-Jamal R, Wallace WA, Harrison DJ. Gene therapy for chronic obstructive pulmonary disease: twilight or triumph? Expert Opin Biol Ther. 2005. 5:333–346.9. Ward C, Forrest IA, Murphy DM, Johnson GE, Robertson H, Cawston TE, et al. Phenotype of airway epithelial cells suggests epithelial to mesenchymal cell transition in clinically stable lung transplant recipients. Thorax. 2005. 60:865–871.10. Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004. 172:1266–1272.11. Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006. 24:686–695.12. Yen CC, Yang SH, Lin CY, Chen CM. Stem cells in the lung parenchyma and prospects for lung injury therapy. Eur J Clin Invest. 2006. 36:310–319.13. Kubo H. Is cell therapy in acute lung injury a realistic dream? Am J Respir Crit Care Med. 2005. 172:794–795.14. Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005. 6:56.15. Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007. 69:1–9.16. Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006. 36:2566–2573.17. Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, et al. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc. 2006. 3:193–207.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- Cell Biological Characteristics of Adult Stem Cells
- The Baiscs and the Prospect of Stem Cells for Pediatric Neurologic Diseases
- Stem Cells and Lung Regeneration